Difference between revisions of "Federal pharmacy law"
From Rx-wiki
m (→Harrison Narcotics Tax Act of 1914) |
m (→Controlled substances prescriptions) |
||
| Line 211: | Line 211: | ||
===Controlled substances prescriptions=== | ===Controlled substances prescriptions=== | ||
| − | Controlled substances have some additional things to keep in mind when reviewing prescription orders. All controlled substance prescriptions require the prescriber to include their DEA number on the prescription. While traditional prescriptions are good for one year from the date they are written and (at the prescriber's discretion) may have a sufficient number of refills to cover an entire one year supply; controlled substances have some differences based on which schedule they are. Schedule V medications may be refilled for up to a one year supply like prescriptions for non-controlled substances. Schedule III-IV medications may be written for up to a six month supply of medications including any refills on the original prescription. Schedule II medications may be written for up to a 90 day supply but may not include any refills. If a patient has a 30 day limit from their insurance the physician would need to write three separate prescriptions for thirty days | + | Controlled substances have some additional things to keep in mind when reviewing prescription orders. All controlled substance prescriptions require the prescriber to include their DEA number on the prescription. While traditional prescriptions are good for one year from the date they are written and (at the prescriber's discretion) may have a sufficient number of refills to cover an entire one year supply; controlled substances have some differences based on which schedule they are. Schedule V medications may be refilled for up to a one year supply like prescriptions for non-controlled substances. Schedule III-IV medications may be written for up to a six month supply of medications including any refills on the original prescription. Schedule II medications may be written for up to a 90 day supply but may not include any refills. If a patient has a 30 day limit from their insurance the physician would need to write three separate prescriptions for thirty days to cover the full 90 days. Prescribers are not allowed to exceed a 90 day supply of a schedule II medications with out seeing the patient again. |
Pharmacies may accept telephone orders and faxes for schedule III-V medications. The pharmacist must immediatley reduce telephone order prescriptions to writing. Schedule II medications may not be ordered over the phone or via a fax machine under ordinary circumstances. | Pharmacies may accept telephone orders and faxes for schedule III-V medications. The pharmacist must immediatley reduce telephone order prescriptions to writing. Schedule II medications may not be ordered over the phone or via a fax machine under ordinary circumstances. | ||
Revision as of 05:05, 10 April 2011
For over a century, federal legislation has been impacting the practice of pharmacy. In almost every case, the purpose of this legislation has been to protect the health, safety, and welfare of the patient from the potential risks of drug use or misuse.
Most of this federal legislation has been initiated in response to issues and concerns at a certain point in time. For example, the Federal Food, Drug, and Cosmetic (FDC) Act passed by Congress was done as a safety concern because of the deaths of over 100 individuals who consumed a drug product containing antifreeze. Other acts that followed also were the result of significant issues with national implications.
While defining pharmacy practice and regulating the profession has primarily been left to the individual states based on the Tenth Amendment to the Constitution, the federal government regulates drug distribution through the Interstate Commerce Clause. This regulation of drug distribution often results, either directly or indirectly, in the regulation of the profession of pharmacy as well. The federal government also has implemented legislation affecting pharmacy practice based on participation in such programs as Medicaid. The counseling provisions of the Omnibus Budget Reconciliation Act, while not directly requiring pharmacist actions, did require the individual state governments to establish expanded standards of practice or risk losing federal funding of their programs. In effect, a back door approach to regulating the profession was utilized.
Over the years, much of the federal legislation that has been passed by Congress has proven itself useful by providing the safety and security that our society has come to expect. Pharmacists have embraced this legislation, albeit sometimes reluctantly, as most legislation has imposed new requirements in such areas as record keeping, counseling, and packaging of pharmaceuticals.
Contents
- 1 Creating a federal law
- 2 Pure Food and Drug Act of 1906
- 3 Harrison Narcotics Tax Act of 1914
- 4 Food, Drug, and Cosmetic Act of 1938
- 5 Durham-Humphrey Amendment of 1951
- 6 Kefauver-Harris Amendment of 1962
- 7 Comprehensive Drug Abuse Prevention and Control Act of 1970 (with updates)
- 8 Occupational Safety and Health Act of 1970
- 9 Poison Prevention Packaging Act of 1970
- 10 Medical Device Amendments of 1976
- 11 Federal Antitampering Act of 1983
- 12 Orphan Drug Act of 1983
- 13 Drug Price Competition and Patent-Term Restoration Act of 1984
- 14 Prescription Drug Marketing Act of 1987
- 15 Omnibus Budget Reconciliation Act of 1987
- 16 Anabolic Steroids Control Act of 1990
- 17 Omnibus Budget Reconciliation Act of 1990
- 18 Dietary Supplement Health and Education Act of 1994
- 19 Uruguay Round Agreements Act of 1994
- 20 Health Insurance Portability and Accountability Act of 1996
- 21 Food and Drug Administration Modernization Act of 1997
- 22 2002 Best Pharmaceuticals for Children Act
- 23 Medicare Modernization Act of 2003
- 24 Combat Methamphetamine Epidemic Act of 2005
- 25 Medicaid Tamper-Resistant Prescription Law of 2007
- 26 Patient Protection and Affordable Care Act of 2010
- 27 See also
- 28 References
Creating a federal law
This is the legislative process as established by President Franklin D. Roosevelt's New Deal administration in 1935.
Under our current systems we have two major routes in which federal legislation may become law:
- The U.S. Congress (House of Representatives and the Senate) passes a law stating objectives to met, or
- a federal agency decides to create or modify a regulation to meet a new situation.
Advanced notice of proposed rule making and/or the proposed rule is published in the Federal Register (FR). The Federal Register is the official journal of the federal government of the United States that contains most routine publications and public notices of government agencies. It is a daily publication excluding holidays.
After public viewing and discussion, any modifications to the rules are also published in the FR, and eventually the final rule will be published in the FR. Once a rule is made official it gets codified into the next edition of the Code of Federal Regulations (CFR). While new regulations are continually becoming effective, the printed volumes of the CFR are only issued annually.
Pure Food and Drug Act of 1906
The original Pure Food and Drug Act (also known as the Wiley Act) was passed by Congress on June 30, 1906 and signed by President Theodore Roosevelt. It prohibited interstate commerce in misbranded and adulterated foods, drinks and drugs under penalty of seizure of the questionable products and/or prosecution of the responsible parties.
To define a couple of terms, to misbrand something means to brand or label misleadingly or fraudulently; while, adulterated means to make impure by adding improper or inferior ingredients.
The Meat Inspection Act was also passed the same day.
Shocking disclosures of insanitary conditions in meat-packing plants, the use of poisonous preservatives and dyes in foods, and cure-all claims for worthless and dangerous patent medicines were the major problems (brought to the public's attention via journalists known as muckrakers) leading to the enactment of these laws. Two of the most famous muckrakers were a journalist named Samuel Hopkins Adams (whom wrote a series of eleven articles for Collier's Weekly, called “The Great American Fraud”) and an author and social activist named Upton Sinclair (whom wrote “The Jungle”)
The following are some important court cases involving the Pure Food and Drug Act:
- In 1911, the U.S. v. Johnson, the Supreme Court ruled that the 1906 Food and Drugs Act does not prohibit false therapeutic claims but only false and misleading statements about the ingredients or identity of a drug.
- In 1912, in reaction to the U.S. v. Johnson verdict, Congress enacted the Shirley Amendment to over come the ruling in U.S. v. Johnson. It prohibited labeling medicines with false therapeutic claims intended to defraud the purchaser, a standard difficult to prove.
- In 1924 in U.S. v. 95 Barrels Alleged Apple Cider Vinegar, the Supreme Court ruled that the Food and Drugs Act condemns every statement, design, or device on a product's label that may mislead or deceive, even if technically true. This case helped to verify the Pure Food and Drug Act's enforceability for dealing with misbranded and adulterated products.
This act is often considered the beginning of the FDA.
Harrison Narcotics Tax Act of 1914
The Harrison Narcotics Tax Act was a United States federal law that regulated and taxed the production, importation, distribution and use of opiates. The act was proposed by Representative Francis Burton Harrison of New York and was approved on December 17, 1914 when it was signed into law by President Woodrow Wilson.
"An act To provide for the registration of, with collectors of internal revenue, and to impose a special tax on all persons who produce, import, manufacture, compound, deal in, dispense, sell, distribute, or give away opium or coca leaves, their salts, derivatives, or preparations, and for other purposes."
This act was intended to curve the growing number of opium addictions within the U.S. As well as to deal with concerns over our new territory in the Philippines.
Following the Spanish-American War the U.S. took over government of the Philippines. Confronted with a licensing system for opium addicts, a Commission of Inquiry was appointed to examine alternatives to this system. The Brent Commission recommended that narcotics should be subject to international control.
This proposal was supported by the United States Department of State and in 1906 President Theodore Roosevelt called for an international opium conference, which was held in Shanghai in 1909. A second conference was held at the Hague in 1911, and out of it came the first international drug control treaty, the International Opium Convention of 1912, aimed primarily at solving the British-caused opium problems of China.
In 1914, the Senate considered the Harrison bill. The act was supported by the Secretary of State William Jennings Bryan who urged that the law be passed to fulfill the obligation of the new international treaty. The debate was about international obligations rather than morality.
The act appears to be concerned about the marketing of opiates. However a clause applying to doctors allowed distribution "in the course of his professional practice only." This clause was interpreted after 1917 to mean that a doctor could not prescribe opiates to an addict, since addiction was not considered a disease. A number of doctors were arrested and some were imprisoned. The medical profession quickly learned not to supply opiates to addicts.
The impact of diminished supply was obvious by mid-1915. A 1918 commission called for sterner law enforcement. Congress responded by tightening up the Harrison Act — the importation of heroin for any purpose was banned in 1924.
This act fell under the regulation of the IRS for tax collection and was enforced by the U.S. Department of Justice.
Food, Drug, and Cosmetic Act of 1938
Because of the shortcomings of the 1906 Act, the FDA petitioned Congress for a new act. Between 1933 and 1937, a legislative battle ensued to replace the 1906 law. On one side of the battle were capitalists calling for laissez faire, on the other were social reformers fighting for government intervention due to concerns over public safety. Ultimately, it was a therapeutic disaster in 1937 that motivated Congress to act. A Tennessee company marketed a sulfa drug in an untested solvent (diethylene glycol) that resulted in the death of 107 people, many of whom were children. The public outcry not only reshaped the drug provisions of the new law to prevent such an event from happening again, but it propelled the bill through Congress.
After being passed by both houses of Congress it was signed into law by President Franklin D. Roosevelt on June 25, 1938
The Federal Food, Drug, and Cosmetic Act contains the following new provisions:
- Extending control to cosmetics and therapeutic devices.
- Requiring new drugs to be shown safe before marketing-starting a new system of drug regulation.
- Eliminating the Shirley Amendment requirement to prove intent to defraud in drug misbranding cases.
- Providing that safe tolerances be set for unavoidable poisonous substances.
- Authorizing standards of identity, quality, and fill-of-container for foods.
- Authorizing factory inspections.
- Adding the remedy of court injunctions to the previous penalties of seizures and prosecutions.
This particular act is still the basis of the Food and Drug Administration's authority although it has received various amendments in the past 75 years. The major amendments include:
- Durham-Humphrey Amendment
- Kefauver Harris Amendment
- Medical Device Amendment
- Orphan Drug Act
- Drug Price Competition and Patent Term Restoration Act
- Prescription Drug Marketing Act
- Dietary Supplements and Health Education Act
- Food and Drug Administration Modernization Act
- Best Pharmaceuticals for Children Act
Durham-Humphrey Amendment of 1951
This amendment to the Food, Drug, and Cosmetic Act, also known as the Prescription Drug Amendment, was signed into law by President Harry S. Truman on October 26, 1951. This amendment was co-sponsored by former vice president and senator Hubert H. Humphrey Jr., who was a pharmacist in South Dakota before beginning his political career. The other sponsor of this amendment was Carl Durham, a pharmacist representing North Carolina in the House of Representatives.
The Durham-Humphrey Amendment, enacted in 1951, resolved the issues left open by the 1938 Act. It established two classes of drugs: Rx legend (prescription) and OTC (over the counter). Prior to the passage of this amendment, drug manufacturers were generally free to determine in which category their drug belonged. A subsection of this amendment granted the FDA the authority to categorize prescription drugs as those that are habit-forming, unsafe for use except under the supervision of a health care practitioner, and/or subject to the new drug application approval process.
Additionally, under this amendment, a drug manufacturer can switch their product from Rx to OTC status by doing any one of the following:
- The drug manufacturer can request the switch by submitting a supplemental application.
- The drug manufacturer can petition the FDA for the switch
- And the primary mechanism for switching drugs to OTC is for the drug manufacturer to go through the OTC process, and a review board which the FDA would have to agree to.
If the FDA agrees, a final report switching from a prescription drug to OTC drug takes place.
The amendment also gave guidance as to what minimal information must be included on the prescription label:
- name and address of the pharmacy,
- serial number of the prescription,
- date of its filling,
- the name of the prescriber,
- the name of the patient,
- the directions for use, and
- any applicable warning labels.
Kefauver-Harris Amendment of 1962
From 1956 to 1962, approximately 10,000 children were born with severe malformities, including phocomelia, because their mothers had taken thalidomide during pregnancy. In 1962, in reaction to the tragedy, the United States Congress enacted the Kefauver-Harris Amendment.
Among other things, the amendment requires drug manufacturers to show the effectiveness of their products as well as their safety, to report adverse events to the FDA, and to ensure that their advertisements to physicians disclose the risks as well as the benefits of their products. Informed consent was required from participants in clinical studies. The FDA also was given jurisdiction over prescription drug advertising. In addition, the agency was required to approve a regulatory submission known as a new drug application before a company could market a new drug and be allowed to issue good manufacturing practice guidelines governing how drugs were to be manufactured. Inspection of drug manufacturers was mandated every 2 years.
The law was signed by President John F. Kennedy on October 10, 1962.
The Kefauver-Harris Amendment provided the modern scenario for approving new drugs:
- Initial drug discovery.
- Preclinical (animal) testing.
- An investigational new drug application (IND) outlines what the sponsor of a new drug proposes for human testing in clinical trials.
- Phase 1 studies (typically involve 20 to 80 healthy people).
- Phase 2 studies (typically involve a few dozen to about 300 people with the ailment that the new drug is intended to treat).
- Phase 3 studies (typically involve several hundred to about 3,000 people with the ailment that the new drug is intended to treat).
- The pre-NDA period, just before a new drug application (NDA) is submitted. A common time for the FDA and drug sponsors to meet.
- Submission of an NDA is the formal step asking the FDA to consider a drug for marketing approval.
- After an NDA is received, the FDA has 60 days to decide whether to file it so it can be reviewed.
- If the FDA files the NDA, an FDA review team is assigned to evaluate the sponsor's research on the drug's safety and effectiveness.
- The FDA reviews information that goes on a drug's professional labeling (information on how to use the drug).
- The FDA inspects the facilities where the drug will be manufactured as part of the approval process.
- FDA reviewers will approve the application or find it either "approvable" or "not approvable."
- Post marketing study commitments, also called Phase 4 commitments.
Comprehensive Drug Abuse Prevention and Control Act of 1970 (with updates)
The Controlled Substances Act (CSA) was passed by Congress as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970. And signed into law by President Richard Nixon on October 27, 1970.
Since the passage of the Harrison Narcotics Tax Act in 1914 many attempts had been made to strengthen government control and regulation of illicit substances with varying degrees of success. The Comprehensive Drug Abuse Prevention and Control Act is our countries most ambitious attempt to date and replaces similar legislation from the past including the Harrison Narcotics Tax Act.
The Comprehensive Drug Abuse Prevention and Control Act provides for the classification, acquisition, distribution, registration/verification of prescribers, and appropriate record keeping requirements of controlled substances. This act also provided the legal framework for the creation of the Drug Enforcement Administration (DEA), which is the organization primarily tasked with the enforcement responsibilities of the CSA.
To ensure an appropriate understanding of this law various updates will be included with discussion of this act.
Classification of controlled substances
Besides over the counter medications (OTC) such as aspirin and ibuprofen, behind the counter medications (BTC) such as Allegra-D (fexofenadine with pseudoephedrine), and prescription medications (Rx legend) such as amoxicillin and digoxin, there is another group of medications to be concerned with called controlled substances.
Controlled substances are medications with further restrictions due to abuse potential. There are 5 schedules of controlled substances with various prescribing guidelines based on abuse potential counter balanced by potential medicinal benefit as determined by the Drug Enforcement Administration and individual state legislative branches. The DEA is provided with this authority by the Controlled Substances Act. Below is a brief explanation of the schedules along with example medications.
Schedule I (CI)
- Characteristics:
- Unaccepted medical use.
- Highest potential for abuse.
- Not available by a prescription.
- Examples:
- LSD
- heroin
- Quaaludes (methaqualone)
Schedule II (CII)
- Characteristics:
- High potential for abuse or misuse.
- Sufficient medicinal use to justify availability as a prescription.
- Examples:
- oxycodone
- morphine
- amphetamines
Schedule III (CIII)
- Characteristics:
- Potential risk for abuse, misuse, and dependence.
- Examples:
- Vicodin (hydrocodone bitartrate and acetaminophen)
- Codeine and codeine containing products in a solid dosage form (tablet, capsule, etc.).
Schedule IV (CIV)
- Characteristics:
- Low potential for abuse and limited risk of dependence.
- Examples:
- phenobarbital
- benzodiazepines
- other sedatives and hypnotics
Schedule V (CV)
- Characteristics:
- Low potential for abuse or misuse.
- Examples:
- Cough medicines that contain a limited amount of codeine.
- Antidiarrheal medications that contain a limited amount of an opiate such as Lomotil (diphenoxylate and atropine).
Many problems associated with drug abuse are the result of legitimately-manufactured controlled substances being diverted from their lawful purpose into the illicit drug traffic. Many of the narcotics, depressants and stimulants manufactured for legitimate medical use are subject to abuse, and have therefore been brought under legal control. The goal of controls is to ensure that these "controlled substances" are readily available for medical use, while preventing their distribution for illicit sale and abuse.
Purchasing
Controlled substances require special consideration when it comes to purchasing.
Schedule III – V drugs may be ordered by a pharmacy or other appropriate dispensary on a general order from a wholesaler and you should check the delivery in against the original order.
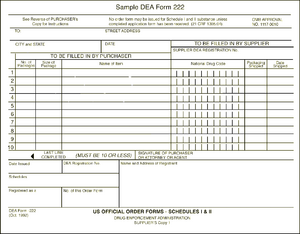
The DEA form 222 is a triplicate form. The pharmacy retains the third sheet while sending the first and second pages to the wholesaler. The wholesaler is responsible for sending the second page to the DEA while retaining the first page for its own records. When the Schedule II medications arrive in the pharmacy they should be checked in against the DEA form.
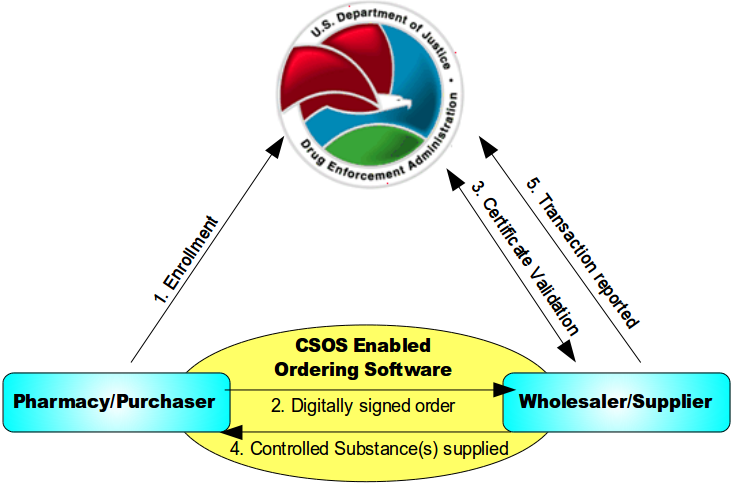
Below is an image explaining how ordering schedule II medications work with a controlled substances ordering system (CSOS)
- An individual enrolls with the DEA and, once approved, is issued a personal CSOS Certificate.
- The purchaser creates an electronic 222 order using an approved ordering software. The order is digitally signed using the purchaser's personal CSOS Certificate and then transmitted to the suppliers. The paper 222 is not required for electronic ordering.
- The supplier receives the purchase order and verifies that the purchaser's certificate is valid with the DEA. Additionally, the supplier validates the electronic order information just like it would a paper order.
- The supplier completes the order and ships to the purchaser. Any communications regarding the order are sent electronically.
- The order is reported by the supplier to the DEA within two business days.
Recieving
When the pharmacy receives controlled substances they should be carefully checked in against the purchase order including product name, quantity, strength, and package size. Controlled substances are shipped in separate containers from the rest of the pharmacy order and should be checked in by a pharmacist, although pharmacy technicians may assist with this process under the direct supervision of a pharmacist. Schedule II medications need to be checked in against your DEA 222 form (whether the paper triplicate form, or the electronic form on your CSOS enabled software).
Controlled substances prescriptions
Controlled substances have some additional things to keep in mind when reviewing prescription orders. All controlled substance prescriptions require the prescriber to include their DEA number on the prescription. While traditional prescriptions are good for one year from the date they are written and (at the prescriber's discretion) may have a sufficient number of refills to cover an entire one year supply; controlled substances have some differences based on which schedule they are. Schedule V medications may be refilled for up to a one year supply like prescriptions for non-controlled substances. Schedule III-IV medications may be written for up to a six month supply of medications including any refills on the original prescription. Schedule II medications may be written for up to a 90 day supply but may not include any refills. If a patient has a 30 day limit from their insurance the physician would need to write three separate prescriptions for thirty days to cover the full 90 days. Prescribers are not allowed to exceed a 90 day supply of a schedule II medications with out seeing the patient again.
Pharmacies may accept telephone orders and faxes for schedule III-V medications. The pharmacist must immediatley reduce telephone order prescriptions to writing. Schedule II medications may not be ordered over the phone or via a fax machine under ordinary circumstances.
DEA has granted three exceptions to the facsimile prescription requirements for Schedule II controlled substances. The facsimile of a Schedule II prescription may serve as the original prescription as follows:
- A practitioner prescribing Schedule II narcotic controlled substances to be compounded for the direct administration to a patient by parenteral, intravenous, intramuscular, subcutaneous or intraspinal infusion may transmit the prescription by facsimile. The pharmacy will consider the facsimile prescription a "written prescription" andno further prescription verification is required.All normal requirements of a legal prescription must be followed.
- Practitioners prescribing Schedule II controlled substances for residents of Long Term Care Facilities (LTCF) may transmit a prescription by facsimile to the dispensing pharmacy. The practitioner’s agent may also transmit the prescription to the pharmacy. The facsimile prescription serves as the original written prescription for the pharmacy.
- A practitioner prescribing a Schedule II narcotic controlled substance for a patient enrolled in a hospice care program certified and/or paid for by Medicare under Title XVIII or a hospice program which is licensed by the state may transmit a prescription to the dispensing pharmacy by facsimile. The practitioner or the practitioner’s agent may transmit the prescription to the pharmacy. The practitioner or agent will note on the prescription that it is for a hospice patient. The facsimile serves as the original written prescription.
For Schedule II controlled substances, an oral order is only permitted in an emergency situation. An emergency situation is defined as a situation in which:
- Immediate administration of the controlled substance is necessary for the proper treatment of the patient.
- No appropriate alternative treatment is available.
- Provision of a written prescription to the pharmacist prior to dispensing is not reasonably possible for the prescribing physician.
In an emergency, a practitioner may call-in a prescription for a Schedule II controlled substance by telephone to the pharmacy, and the pharmacist may dispense the prescription provided that the quantity prescribed and dispensed is limited to the amount adequate to treat the patient during the emergency period. The prescribing practitioner must provide a written and signed prescription to the pharmacist within seven days. Further, the pharmacist must notify DEA if the prescription is not received.
The regulations concerning electronic transmission of controlled substances via e-prescribing recently changed. As of June 1, 2010 physicians and pharmacies are now allowed to transmit prescriptions for Schedule II, III, IV, and V medications as long as they are using properly certified software (i.e., SureScripts). While this is a recent shift in federal law, some states may still prohibit e-prescribing for controlled substances.
DEA number verification
Many problems associated with drug abuse are the result of legitimately-manufactured controlled substances being diverted from their lawful purpose into the illicit drug traffic. Many of the narcotics, depressants and stimulants manufactured for legitimate medical use are subject to abuse, and have therefore been brought under legal control. The goal of controls is to ensure that these "controlled substances" are readily available for medical use, while preventing their distribution for illicit sale and abuse.
Under federal law, all businesses which manufacture or distribute controlled drugs, all health professionals entitled to dispense, administer or prescribe them, and all pharmacies entitled to fill prescriptions must register with the DEA. Authorized registrants receive a "DEA number". Registrants must comply with a series of regulatory requirements relating to drug security, records accountability, and adherence to standards.
A DEA number is a series of numbers assigned to a health care provider (such as a dentist, physician, nurse practitioner, or physician assistant) allowing them to write prescriptions for controlled substances. Legally the DEA number is solely to be used for tracking controlled substances. The DEA number, however, is often used by the industry as a general "prescriber" number that is a unique identifier for anyone who can prescribe medication.
A valid DEA number consists of:
- 2 letters and 7 digits
- The first letter is always an A (deprecated), B (most common), or F (new) for a dispenser. (Also M is available for mid-level practitioners in some states and either P or R is used for a wholesaler)
- The second letter is the initial of the registrant's last name
- The seventh digit is a "checksum" that is calculated as:
- Add together the first, third and fifth digits
- Add together the second, fourth and sixth digits and multiply the sum by 2
- Add the above 2 numbers
- The last digit (the ones value) of this last sum is used as the seventh digit in the DEA number
Record keeping requirements
Every pharmacy must maintain complete and accurate records on a current basis for each controlled substance purchased, received, distributed, dispensed, or otherwise disposed of. These records must be maintained for 2 years. It is also required that records and inventories of Schedule II and Schedule III, IV, and V drugs must be maintained separately from all other records or be in a form that is readily retrievable from other records. The "readily retrievable" requirement means that records kept by automatic data processing systems or other electronic means must be capable of being separated out from all other records in a reasonable time. In addition, some notation, such as a 'C' stamp, an asterisk, red line, or other visually identifiable mark must distinguish controlled substances from other items.
Inventory requirements
A pharmacy is required by the DEA to take an inventory of controlled substances every 2 years (biennially). This inventory must be done on any date that is within 2 years of the previous inventory date. The inventory record must be maintained at the registered location in a readily retrievable manner for at least 2 years for copying and inspection by the Drug Enforcement Administration. An inventory record of all Schedule II controlled substances must be kept separate from those of other controlled substances. Submission of a copy of any inventory record to the DEA is not required unless requested.
When taking the inventory of Schedule II controlled substances, an actual physical count must be made. For the inventory of Schedule III, IV, and V controlled substances, an estimate count may be made. If the commercial container holds more than 1000 dosage units and has been opened, however, an actual physical count must be made.
Occupational Safety and Health Act of 1970
Poison Prevention Packaging Act of 1970
Medical Device Amendments of 1976
Federal Antitampering Act of 1983
Orphan Drug Act of 1983
Drug Price Competition and Patent-Term Restoration Act of 1984
Prescription Drug Marketing Act of 1987
Omnibus Budget Reconciliation Act of 1987
Anabolic Steroids Control Act of 1990
Omnibus Budget Reconciliation Act of 1990
Dietary Supplement Health and Education Act of 1994
Uruguay Round Agreements Act of 1994
Health Insurance Portability and Accountability Act of 1996
Food and Drug Administration Modernization Act of 1997
2002 Best Pharmaceuticals for Children Act
Medicare Modernization Act of 2003
Combat Methamphetamine Epidemic Act of 2005
Medicaid Tamper-Resistant Prescription Law of 2007
Patient Protection and Affordable Care Act of 2010
See also
References
- Pharmacy Times, A Review of Federal Legislation Affecting Pharmacy Practice, Virgil Van Dusen , RPh, JD and Alan R. Spies , RPh, MBA, JD, PhD, https://secure.pharmacytimes.com/lessons/200612-01.asp
- Strauss's Federal Drug Laws and Examination Review, Fifth Edition (revised), Steven Strauss, CRC Press, 2000
- Food and Drug Administration, Legislation, http://www.fda.gov/RegulatoryInformation/Legislation/default.htm
- Food and Drug Administration, History, http://www.fda.gov/AboutFDA/WhatWeDo/History/default.htm
- Wikipedia, Durham-Humphrey Amendment, http://en.wikipedia.org/wiki/Durham-Humphrey_Amendment
- Wikipedia, Kefauver-Harris Amendment, http://en.wikipedia.org/wiki/Kefauver_Harris_Amendment
- Food and Drug Administration, The FDA's Drug Review Process: Ensuring Drugs Are Safe and Effective, http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143534.htm
- Pharmacy Times, An Overview and Update of the Controlled Substances Act of 1970, Virgil Van Dusen , RPh, JD and Alan R. Spies , RPh, MBA, JD, PhD, https://secure.pharmacytimes.com/lessons/200702-01.asp
- Drug Enforcement Administration Office of Diversion Control, Title 21 United States Code (USC) Controlled Substances Act, http://www.deadiversion.usdoj.gov/21cfr/21usc/index.html
- Controlled Substance Ordering System, http://www.deaecom.gov/
- Drug Enforcement Administration Office of Diversion Control, Practitioner's Manual SECTION V – VALID PRESCRIPTION REQUIREMENTS, http://www.deadiversion.usdoj.gov/pubs/manuals/pract/section5.htm