Difference between revisions of "Anabolic Steroids Control Act"
From Rx-wiki
Kgambridge (Talk | contribs) (Created page with "300pxAnabolic steroids are defined as synthetic derivatives of the male hormone testosterone, having pronounced anabolic properties and relatively we...") |
(→See also) |
||
| Line 8: | Line 8: | ||
==See also== | ==See also== | ||
| − | [[Federal pharmacy law]] | + | [[Federal pharmacy law]]<br/> |
| + | [[Drug Enforcement Administration]] | ||
==References== | ==References== | ||
Revision as of 09:55, 15 June 2011
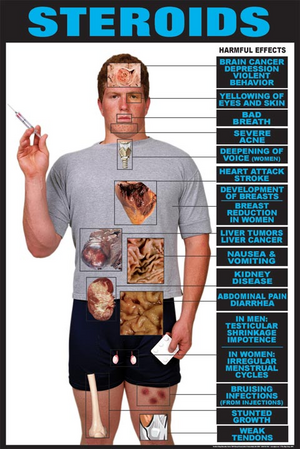
Anabolic steroids are defined as synthetic derivatives of the male hormone testosterone, having pronounced anabolic properties and relatively weak andronergic properties (i.e., producing masculine characteristics), which are used clinically mainly to promote growth and to repair body tissue in senility, debilitating illness, and convalescence. Anabolic steroids may also be referred to as andronergic-anabolic steroids.The intent of the ASCA is to minimize (or eliminate) the use of anabolic steroids for non-medical purposes. Prior to the ASCA, anabolic steroids were regulated as drugs pursuant to the FDCA.
The DEA has classified anabolic steroids as Schedule III controlled substances; however, certain products which are considered to have no significant potential for abuse because of their concentration, preparation, mixture, or delivery system, are exempt from being classified as control substances (such as Premarin with methyltestosterone tablets).
In addition, the classification does not include anabolic steroids expressly intended for cattle or other non-human species and which are approved by the FDA. However, prescribing, dispensing or distributing such substances for other than implantation in cattle or other non-human species is a violation of the law.
See also
Federal pharmacy law
Drug Enforcement Administration