Difference between revisions of "Drug Enforcement Administration"
From Rx-wiki
(Created page with "The Drug Enforcement Administration (DEA) is a United States Department of Justice law enforcement agency, a federal police service tasked with enforcing the Controlled Substance...") |
|||
| Line 20: | Line 20: | ||
==Genealogy== | ==Genealogy== | ||
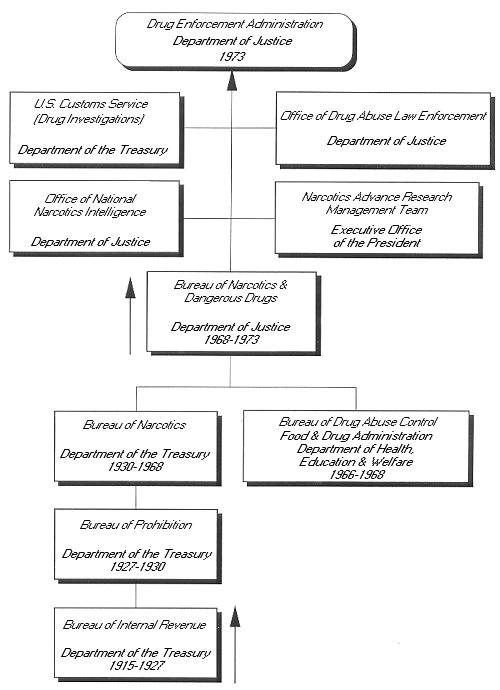
[[Image:genealogy.gif]] | [[Image:genealogy.gif]] | ||
| + | |||
| + | ==Medication Schedules== | ||
| + | Controlled substances are medications with further restrictions due to abuse potential. There are 5 schedules of controlled substances with various prescribing guidelines based on abuse potential counter balanced by potential medicinal benefit as determined by the Drug Enforcement Administration and individual state legislative branches. The DEA is provided with this authority by the Controlled Substances Act. Below is a brief explanation of the schedules along with example medications. | ||
| + | |||
| + | '''Schedule I''' (''CI'') | ||
| + | *Characteristics: | ||
| + | **Unaccepted medical use. | ||
| + | **Highest potential for abuse. | ||
| + | **Not available by a prescription. | ||
| + | *Examples: | ||
| + | **LSD | ||
| + | **heroin | ||
| + | **Quaaludes (methaqualone) | ||
| + | '''Schedule II''' (''CII'') | ||
| + | *Characteristics: | ||
| + | **High potential for abuse or misuse. | ||
| + | **Sufficient medicinal use to justify availability as a prescription. | ||
| + | *Examples: | ||
| + | **oxycodone | ||
| + | **morphine | ||
| + | **amphetamines | ||
| + | '''Schedule III''' (''CIII'') | ||
| + | *Characteristics: | ||
| + | **Potential risk for abuse, misuse, and dependence. | ||
| + | *Examples: | ||
| + | **Vicodin (hydrocodone bitartrate and acetaminophen) | ||
| + | **Codeine and codeine containing products in a solid dosage form (tablet, capsule, etc.). | ||
| + | '''Schedule IV''' (''CIV'') | ||
| + | *Characteristics: | ||
| + | **Low potential for abuse and limited risk of dependence. | ||
| + | *Examples: | ||
| + | **phenobarbital | ||
| + | **benzodiazepines | ||
| + | **other sedatives and hypnotics | ||
| + | '''Schedule V''' (''CV'') | ||
| + | *Characteristics: | ||
| + | **Low potential for abuse or misuse. | ||
| + | *Examples: | ||
| + | **Cough medicines that contain a limited amount of codeine. | ||
| + | **Antidiarrheal medications that contain a limited amount of an opiate such as Lomotil (diphenoxylate and atropine). | ||
| + | |||
| + | '''CI medications''' are not available via a prescription <br/> | ||
| + | '''CII medications''' may be written for a maximum 90 day supply without refills<br/> | ||
| + | '''CIII – CV medications''' may be written for up to a 6 month supply | ||
| + | |||
| + | Many problems associated with drug abuse are the result of legitimately-manufactured controlled substances being diverted from their lawful purpose into the illicit drug traffic. Many of the narcotics, depressants and stimulants manufactured for legitimate medical use are subject to abuse, and have therefore been brought under legal control. The goal of controls is to ensure that these "controlled substances" are readily available for medical use, while preventing their distribution for illicit sale and abuse. | ||
==See also== | ==See also== | ||
Revision as of 17:22, 4 October 2011
The Drug Enforcement Administration (DEA) is a United States Department of Justice law enforcement agency, a federal police service tasked with enforcing the Controlled Substances Act of 1970. Not only is the DEA the lead agency for domestic enforcement of federal drug laws (sharing concurrent jurisdiction with the Federal Bureau of Investigation), it also has sole responsibility for coordinating and pursuing U.S. drug investigations abroad.
The DEA was created by President Richard Nixon through an Executive Order in July 1973 in order to establish a single unified command to combat "an all-out global war on the drug menace." At its outset, the DEA had 1,470 Special Agents and a budget of less than $75 million. Furthermore, in 1974, the DEA had 43 foreign offices in 31 countries. Today, the DEA has approximately 5,000 Special Agents, a budget of more than $2 billion and 86 foreign offices in 62 countries.
Mission Statement
The mission of the DEA is to enforce the controlled substances laws and regulations of the United States and bring to the criminal and civil justice system of the United States, or any other competent jurisdiction, those organizations and principal members of organizations, involved in the growing, manufacture, or distribution of controlled substances appearing in or destined for illicit traffic in the United States; and to recommend and support non-enforcement programs aimed at reducing the availability of illicit controlled substances on the domestic and international markets.
In carrying out its mission as the agency responsible for enforcing the controlled substances laws and regulations of the United States, the DEA's primary responsibilities include:
- Investigation and preparation for the prosecution of major violators of controlled substance laws operating at interstate and international levels.
- Investigation and preparation for prosecution of criminals and drug gangs who perpetrate violence in our communities and terrorize citizens through fear and intimidation.
- Management of a national drug intelligence program in cooperation with federal, state, local, and foreign officials to collect, analyze, and disseminate strategic and operational drug intelligence information.
- Seizure and forfeiture of assets derived from, traceable to, or intended to be used for illicit drug trafficking.
- Enforcement of the provisions of the Controlled Substances Act as they pertain to the manufacture, distribution, and dispensing of legally produced controlled substances.
- Coordination and cooperation with federal, state and local law enforcement officials on mutual drug enforcement efforts and enhancement of such efforts through exploitation of potential interstate and international investigations beyond local or limited federal jurisdictions and resources.
- Coordination and cooperation with federal, state, and local agencies, and with foreign governments, in programs designed to reduce the availability of illicit abuse-type drugs on the United States market through nonenforcement methods such as crop eradication, crop substitution, and training of foreign officials.
- Responsibility, under the policy guidance of the Secretary of State and U.S. Ambassadors, for all programs associated with drug law enforcement counterparts in foreign countries.
- Liaison with the United Nations, Interpol, and other organizations on matters relating to international drug control programs.
Genealogy
Medication Schedules
Controlled substances are medications with further restrictions due to abuse potential. There are 5 schedules of controlled substances with various prescribing guidelines based on abuse potential counter balanced by potential medicinal benefit as determined by the Drug Enforcement Administration and individual state legislative branches. The DEA is provided with this authority by the Controlled Substances Act. Below is a brief explanation of the schedules along with example medications.
Schedule I (CI)
- Characteristics:
- Unaccepted medical use.
- Highest potential for abuse.
- Not available by a prescription.
- Examples:
- LSD
- heroin
- Quaaludes (methaqualone)
Schedule II (CII)
- Characteristics:
- High potential for abuse or misuse.
- Sufficient medicinal use to justify availability as a prescription.
- Examples:
- oxycodone
- morphine
- amphetamines
Schedule III (CIII)
- Characteristics:
- Potential risk for abuse, misuse, and dependence.
- Examples:
- Vicodin (hydrocodone bitartrate and acetaminophen)
- Codeine and codeine containing products in a solid dosage form (tablet, capsule, etc.).
Schedule IV (CIV)
- Characteristics:
- Low potential for abuse and limited risk of dependence.
- Examples:
- phenobarbital
- benzodiazepines
- other sedatives and hypnotics
Schedule V (CV)
- Characteristics:
- Low potential for abuse or misuse.
- Examples:
- Cough medicines that contain a limited amount of codeine.
- Antidiarrheal medications that contain a limited amount of an opiate such as Lomotil (diphenoxylate and atropine).
CI medications are not available via a prescription
CII medications may be written for a maximum 90 day supply without refills
CIII – CV medications may be written for up to a 6 month supply
Many problems associated with drug abuse are the result of legitimately-manufactured controlled substances being diverted from their lawful purpose into the illicit drug traffic. Many of the narcotics, depressants and stimulants manufactured for legitimate medical use are subject to abuse, and have therefore been brought under legal control. The goal of controls is to ensure that these "controlled substances" are readily available for medical use, while preventing their distribution for illicit sale and abuse.
See also
Federal pharmacy law
Comprehensive Drug Abuse Prevention and Control Act
Combat Methamphetamine Epidemic Act
Food and Drug Administration
References
- DEA, DEA History, http://www.justice.gov/dea/history.htm
- DEA, DEA Staffing and Budget, http://www.justice.gov/dea/agency/staffing.htm
- DEA, DEA Mission Statement, http://www.justice.gov/dea/agency/mission.htm
- DEA, Genealogy, http://www.justice.gov/dea/agency/genealogy.htm