Compounding
From Rx-wiki
this article is currently being revised, please be patient
This article is intended to provide a broad overview of sterile and nonsterile Compounding. This article will cover the following knowledge areas:
- Infection control (e.g., hand washing, PPE)
- Handling and disposal requirements (e.g., receptacles, waste streams)
- Documentation (e.g., batch preparation, compounding record)
- Determine product stability (e.g., beyond use dating, signs of incompatibility)
- Selection and use of equipment and supplies
- Sterile compounding processes
- Non-sterile compounding processes
- Terminology
Contents
- 1 Terminology
- 2 Secundum artem
- 3 Basic steps for preparing compounded drug preparations
- 4 Safety
- 5 Documentation
- 6 Product stability
- 7 Nonsterile compounding
- 8 Sterile compounding
- 9 Calculations often used for compounding
- 10 old portion
- 11 Categories of nonsterile compounding
- 12 Basic steps for preparing compounded drug preparations
- 13 Legal issues
- 14 Lab safety
- 15 Equipment
- 16 Weighing and measuring
- 17 Definitions
- 18 Compounded dosage forms
- 19 Labeling
- 20 See also
- 21 References
Terminology
To get started in this article, there are some terms that should be defined.
extemporaneous compounding - Extemporaneous compounding can be defined as the preparation, mixing, assembling, packaging, and labeling of a drug product based on a prescription from a licensed practitioner for the individual patient in a form that the drug is not readily available in (extemporaneous = impromptu, compounding = the act of combining things).
United States Pharmacopeia (USP) - The United States Pharmacopeia (USP) is the official pharmacopeia of the United States, published dually with the National Formulary as the USP-NF. Prescriptions and over-the-counter medicines and other healthcare products sold in the United States are required to follow the standards in the USP-NF. The USP also sets for food ingredients and dietary supplements. Chapters in the USP that are listed as below 1000 are considered enforceable, while chapters enumerated as 1000 or greater are considered guidelines. Therefore, USP 797 and USP 795 are considered enforceable, while USP 1075 and USP 1160 are simply considered guidelines for best practices.
USP 795 - USP Chapter 795, Pharmaceutical Compounding-Nonsterile Preparations, codifies the rules pharmacists and pharmacy technicians must follow when compounding nonsterile formulations intended for humans and animals.
USP 797 - USP Chapter 797, Pharmaceutical Compounding-Sterile Preparations, provides the first set of enforceable sterile compounding standards issued by the United States Pharmacopeia (USP). USP Chapter 797 describes the procedures and requirements for compounding sterile preparations and sets the standards that apply to all settings in which sterile preparations are compounded.
USP 1075 - USP Chapter 1075, Good Compounding Practices, is intended to provide guidelines on applying best practices in compounding, both sterile and nonsterile.
USP 1160 - USP Chapter 1160, Pharmaceutical Calculations in Prescription Compounding, provides general information on the mathematical concepts required for compounding pharmaceutical preparations.
The Joint Commission (TJC) - The Joint Commission, formerly the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) is a nonprofit organization that accredits more than 20,000 healthcare organizations and programs in the United States.
Board of Pharmacy (BOP) - Each state has their own board of pharmacy. The BOP sets standards, roles, and requirements for pharmacy personnel and practice setting in their state.
Occupational Safety and Health Administration (OSHA) - OSHA is a government agency within the United States Department of Labor responsible for maintaining safe and healthy work environments.
Material Safety Data Sheet (MSDS) - OSHA-required notices on hazardous substances which provide hazard, handling, clean-up, and first aid information.
personal protective equipment (PPE) - Personal protective equipment is worn by an individual to provide both protection to the wearer from the environment or specific items they are manipulating, and to prevent exposing the environment or the items being manipulated directly to the wearer of the PPE.
nosocomial infection - A nosocomial infection is an infection acquired while in the hospital.
batch preparation - A batch preparation is one in which multiple identical units are prepared in a single operation in anticipation of prescriptions.
trituration - Trituration is a method to reduce particle size (comminution); it may also include the grinding together of two or more substances in a mortar to mix them as you should want a fine powder to make incorporation better. Trituration is achieved by firmly holding the pestle and exerting a downward pressure with it while moving it in successively larger circles starting at the center of the mortar, moving outward to the side of the mortar, then back again toward the center.
spatulation - Spatulation is the mixing of powders and semi-solids (ointments, creams, etc.) on an ointment pad or slab using a spatula. With this method there is no particle size reduction, so the powders to be mixed must be fine and of uniform size.
geometric dilution - Geometric dilution, also called geometric incorporation, is the process by which a homogeneous mixture of even distribution of two or more substances is achieved. When using this method, the smallest quantity of active ingredient is mixed thoroughly with a proportion quantity of the diluent or base on the ointment slab. More diluent (base) is added in amounts proportionate to the volume of the mixture on the ointment slab. This process is repeated until all of the ingredients are incorporated in the mixture.
levigation - Levigation is the process of reducing particle size of a solid by triturating it in a mortar or spatulating it on an ointment slab or pad with a small amount of a liquid called a levigating agent. Levigating agents make incorporating solids easier, and they make a smooth, elegant preparation.
parenteral - A parenteral can be defined as any administration route not involving the GI tract, but it is used more commonly used in reference to routes that require sterile preparations (injectable routes, ophthalmic, and inhalation).
compounded sterile preparations (CSP) - Compounded sterile preparations are admixtures that need to be assembled under aseptic conditions to prevent contamination.
aseptic techniques - Aseptic techniques are techniques or methods that maintain the sterile condition of products.
pyrogens - Pyrogens are chemicals produced by microorganisms that can cause pyretic (fever) reactions in patients
precipitate - A precipitate is an insoluble substance separated from a solution due to a reaction between incompatible substances.
osmolarity - Osmolarity, also called osmotic pressure, is a characteristic of a solution determined by the number of dissolved particles in it.
isotonic - An isotonic solution has an osmolarity equivalent to that of blood
hypertonic - A hypertonic solution has a greater osmolarity than that of blood
hypotonic - A hypotonic solution has a lesser osmolarity than that of blood
ions - Ions are molecular particles that carry electric charges.
additive - An additive, in the context of sterile compounding, is a drug that is added to a parenteral solution.
admixture - An admixture is the resulting solution when a drug is added to a parenteral solution.
lyophlized - Lyophilized powders are simply freeze-dried powders.
diluent - A diluent is a solvent that dissolves a lyophilized powder or dilutes a solution.
coring - Coring occurs when a needle damages the rubber closure of a parenteral container causing fragments of the closure to fall into the container and contaminate its contents.
HEPA filter - A HEPA filter is a high efficiency particulate air filter.
laminar airflow - Laminar airflow provides continuous air movement at a uniform rate in one direction.
ISO Class - The International Organization for Standardization has established various levels of air cleanliness. The lower the number the fewer particles that are suspended in it and the cleaner the air is. The ISO classes commonly discussed in sterile compounding are ISO Class 5 (3,520 particles of 0.5m micron or larger in a cubic meter), ISO Class 7 (352,000 particles of 0.5m micron or larger in a cubic meter), and ISO Class 8 (3,520,000 particles of 0.5m micron or larger in a cubic meter).
parenteral nutrition (PN) - Parenteral nutrition is a complex solutions with two base solutions (amino acids and dextrose) and additional micronutrients. Lipids may or may not also be present in parenteral nutrition.
automated compounding device (ACD) - An automated compounding device is a machine used to prepare CSPs (e.g., PN).
hazardous drugs - Hazardous drugs are drugs that are known to cause genotoxicity, which is the ability to cause a change or mutation in genetic material; carcinogenicity, the ability to cause cancer in animal models, humans or both; teratogenicity, which is the ability to cause defects on fetal development or fetal malformation; and lastly hazardous drugs are known to have the potential to cause fertility impairment, which is a major concern for most clinicians. These drugs can be classified as antineoplastics, cytotoxic agents, biologic agents, antiviral agents and immunosuppressive agents.
antineoplastic agents - Antineoplastic agents are drugs that inhibit and combat the development of tumors.
ante area - An area that is ISO class 8 or better where hand hygiene and garbing is performed. It also functions as a transition area between the buffer area and the rest of the facility.
buffer area - An area that is required to be ISO class 7 or better. A primary engineerirng control (PEC) such as a laminar airflow workbench would be located in this area.
primary engineering control (PEC) - A primary engineering control, which could include a room or device, provides an ISO Class 5 environment for compounding sterile products. Examples of primary engineering control may include laminar airflow workbenches (LAFW), compounding aseptic isolators (CAI), biological safety cabinets (BSC), compounding aseptic containment isolators (CACI), and clean rooms that create an ISO Class 5 environment.
positive pressure room - A room that has higher pressure than adjacent spaces and therefore has a net flow of air out of the room.
negative pressure room - If a pharmacy prepares more than a low volume of hazardous drugs the appropriate type of PEC should be located within a negative pressure room. A negative pressure room has a has a lower pressure than adjacent rooms and therefore the net flow of air is into the room.
Secundum artem
Extemporaneous compounding, often just called compounding, is a necessary skill for many pharmacists and pharmacy technicians. Many compounders will use the Latin term secundum artem (according to the art) when referring to the skills required to prepare these products. Extemporaneous compounding can be defined as the preparation, mixing, assembling, packaging, and labeling of a drug product based on a prescription from a licensed practitioner for the individual patient in a form that the drug is not readily available (extemporaneous = impromptu, compounding = the act of combining things). Extemporaneous compounding is required for prescription orders that are not commercially available in the requested strength or dosage form.
There are two types of pharmaceutical compounding, sterile (ophthalmics, IVs, parenteral nutrition, chemotherapy, and various other injectables) and nonsterile (creams, ointments, oral suspensions, capsules, suppositories, medication sticks, troches, etc.).
Basic steps for preparing compounded drug preparations
Regardless of the compounding category, there is a general order of work for all extemporaneous compounding. By following a procedure you will be able to work more effectively.
- Carefully read and interpret the prescription or medication order.
- Note any missing or confusing information; clarify, gather, and add this information to the drug order.
- Check the dose,dosage regimen, dosage form, and route of administration for appropriateness.
- Determine a preliminary compounding procedure.
- Perform necessary calculations. If possible have a colleague check calculations.
- Select required ingredients. It is the responsibility of the pharmacist to choose appropriate quality ingredients for compounding. The preferred grade for this is USP or NF. If an official USP or NF ingredient is not available, the pharmacist should use professional judgment in the selection of an alternative source so that the safety and purity of the ingredient is assured. This may require requesting a certificate of analysis from the supplier.
- Choose appropriate compounding equipment.
- Using recommended techniques, prepare the product.
- A visual inspection of the product should be done.
- Choose an appropriate container and package the preparation.
- Determine an appropriate beyond use date
- Label container and include recommended auxiliary labels
- Document the compounding process.
Safety
Extemporaneous compounding brings with it its own safety concerns. There is a vast array of information to be aware of with safety requirements depending as to what kinds of chemicals you are working with. Of particular concern is infection control through proper hand hygiene and personal protective equipment, and proper handling with regard to disposal and waste management.
Infection control
Infection control is concerned with preventing nosocomial or healthcare-associated infection. Infection control addresses factors related to the spread of infections within the healthcare setting (whether patient-to-patient, from patients to staff and from staff to patients, or among-staff), including prevention via hand hygiene and through the use of personal protective equipment (PPE).
When assembling CSPs, preparers should don personal protective equipment (PPE) to further minimize the likelihood that they will contaminate the final product. USP 797 provides requirements for how personnel should wash and garb including the order that they should don their PPE, from dirtiest to cleanest. First, personnel should remove unnecessary outer garments and visible jewelry (scarves, rings, ear rings, etc.). Next, they should don shoe covers, facial hair and hair covers, and masks (optionally they may include a face shield). Next, they must perform appropriate hand hygiene.
USP 797 states:
"...personnel perform a thorough hand-cleansing procedure by removing debris from under fingernails using a nail cleaner under running warm water followed by vigorous hand and arm washing to the elbows for at least 30 seconds with either nonantimicrobial or antimicrobial soap and water."
After hand washing, a nonshedding gown with sleeves that fit snugly around the wrists should be put on. Once inside the buffer area an antiseptic hand cleansing using a waterless alcohol-based hand scrub should be performed. Sterile gloves are the last item donned prior to compounding.
Disposal
Proper waste stream management is very important in pharmacy as mismanagement can have negative impacts on the community, the environment, and can cause fines to levied against a pharmacy or health care facility. Waste that poses no ecological threats, is devoid of protected heath information, and poses no major safety risks may be disposed of in regular waste receptacles.
Broken ampules, syringes, and various needles need to be treated with caution to avoid accidental needle sticks and/or cuts. If the medications or diluents that these sharps were exposed to are not classified as hazardous, than you may dispose of this waste in red sharps containers.
Properly labeled, leak-proof, and spill-proof containers of nonreactive plastic are required for areas where hazardous waste is generated, and can be further broken down into either yellow or black containers. Fully used vials, syringes, tubing, and bags of hazardous drug waste along with PPE used while working with hazardous drugs may be disposed in yellow, properly labeled containers. Also, any partially used or expired hazardous drugs that are not also considered to be RCRA (Resource Conservation and Recovery Act) regulated hazardous drugs should be placed in these yellow buckets. Trace contaminated items such as booties, gowns, and masks, even if they were not involved in spills, MUST be treated as hazardous waste (the yellow buckets are sufficient for this).
Partially used bags, vials, syringes, etc. of RCRA listed hazardous pharmaceutical waste MUST be placed in black RCRA approved containers. Hazardous waste must be properly manifested and transported by a federally permitted hazardous waste transporter to a federally permitted hazardous waste storage, treatment, or disposal facility.
Documentation
Documentation of extemporaneously compounded medications is an important responsibility within the pharmacy as it provides a chain of accountability for the preparations and it aids in tracking drug recalls. Documentation can be broken into two major categories, record keeping and labeling.
Record keeping
Whenever preparing any extemporaneous compound, the minimum information to maintain would be a method for tracking whom the compounder(s) is/are, whom verified that the procedures for compounding were carried out correctly, and drugs/chemical used including manufacturer, lot number, and expiration date, and an internally assigned batch number and beyond use date. Many facilities will also track information on all the equipment used in preparing the compound.
Sometimes facilities will compound batch preparations. A batch preparation is one in which multiple identical units are prepared in a single operation in anticipation of prescriptions. Some examples of batch preparations might include preparing 100 capsules for hormone replacement therapy when only 30 have been ordered, or preparing 10 IV minibags of the same drug at the same concentration from a bulk container when only two have been ordered. These preparations are being made in anticipation of additional orders being received in the pharmacy. Since none of these medications will be dispensed or sold without a specific patient in mind, it does not cross the line from compounding to manufacturing. Documentation must also be maintained on all of these batch preparations as well.
Labeling
An important aspect of documentation is providing proper labeling of compounded preparations for others to read once dispensed from the pharmacy. While the requirements and expectations of what kind of labeling information should be present on a medication dispensed to a patient for use at home will vary from those intended for use in a institutional setting there are some expectations and requirements of both. The active ingredients and concentrations should be present as well as a tracking number (whether a batch number or prescription number) a verification of whom prepared and checked the preparation (even if just listed by initials), and an appropriate beyond use date.
Product stability
A compounded medication needs to be stable for its intended purpose. This includes both concerns over chemical compatibility and labeling the product with an appropriate beyond use date
Chemical compatibility
Chemical compatibility is a measure of how stable a substance is when mixed with another substance. Some incompatibilities are visible (color change or formation of a precipitate) and other chemical incompatibilities can be equally concerning even if not visible such as the formation of gases and volatile chemicals. An example of a chemical incompatibility is the formation of a calcium phosphate precipitate that falls out of solution when calcium gluconate and potassium phosphate are mixed together in sufficient concentration.
Beyond use date
The requirement to provide a beyond use date sometimes causes confusion as there is some ambiguity between what an expiration date is and what a beyond-use date is. An expiration date is the date put on the label of a drug product by the manufacturer or distributor of the product. Federal law requires that manufactured (compounding is not considered manufacturing) drug products be labeled with an expiration date. The beyond use date is the date placed on a prescription bottle by a pharmacy noting when that prescription should no longer be used.
The American Medical Association (AMA) states:
"The beyond-use date placed on the label shall be no later than the expiration date on the manufacturer’s container. The beyond-use date is a date after which an article [drug] must not be used. Based on the information supplied by the manufacturer, the dispenser shall place on the label of the prescription container a suitable beyond-use date to limit the patient’s use of the article."
For water containing formulations, prepared from ingredients in solid form, the beyond use date is not later than 14 days when stored at cold temperatures. Generally, a two-week dating is the maximum for ointments containing water if a preservative is not present. Ointment with water are subject to microbial growth. Ointments without water can have much longer dating. Ointment jars, although widely used, expose the preparation to air and microbial contamination when opened and when the ointment is removed using the fingers. The maximum beyond use date for a very stable preparation would be 6 months or 25% of the time remaining between the time of compounding and the shortest expiration date of the ingredients, whichever is earlier. You need to consult available literature if possible and professional judgment is required in these cases. For all other formulations, the beyond use date is not later than the intended duration of therapy or 30 days, whichever is earlier.
Nonsterile compounding
Compounding pharmacies are capable of preparing a broad array of dosage forms including capsules, tablets, oral suspensions and solutions, suppositories, troches (lozenges), various topical dosage forms (gels, lotions, ointments, creams), lollipops, and more. One of the things that tends to make compounding both interesting and difficult is finding the best way to manipulate a dosage form to benefit a patient.
USP Chapter 795, Pharmaceutical Compounding-Nonsterile Preparations, codifies the rules pharmacists and pharmacy technicians must follow when compounding nonsterile formulations intended for humans and animals. This chapter provides minimal standards for equipment, supplies, labeling, beyond use dates, compounding processes, and definitions of various dosage forms.
USP 1075 has designated three categories of compounding related to different levels of required experience, training, and physical facilities. The considerations factoring into this are:
- the difficulty/complexity of the compound,
- product stability and warnings,
- packaging and storage requirements,
- the physical dosage forms,
- the complexity of calculations required,
- local versus systemic effects of the product,
- risk to the compounder, and
- risk to the patient.
These categories are broken into Simple, Moderate, and Complex compounding. Simple compounding involves preparing compounds from USP monographs and peer-reviewed journals or even just reconstituting and manipulating commercial products within manufacturer guidelines. Required procedures and equipment are well documented. Moderate compounding requires special calculations and procedures and is generally not as well documented as Simple compounding. Complex compounding requires special training, environment, facilities, equipment, and procedures.
Equipment and supplies
Below is a list of equipment commonly used for nonsterile compounding.
Mortar and pestle
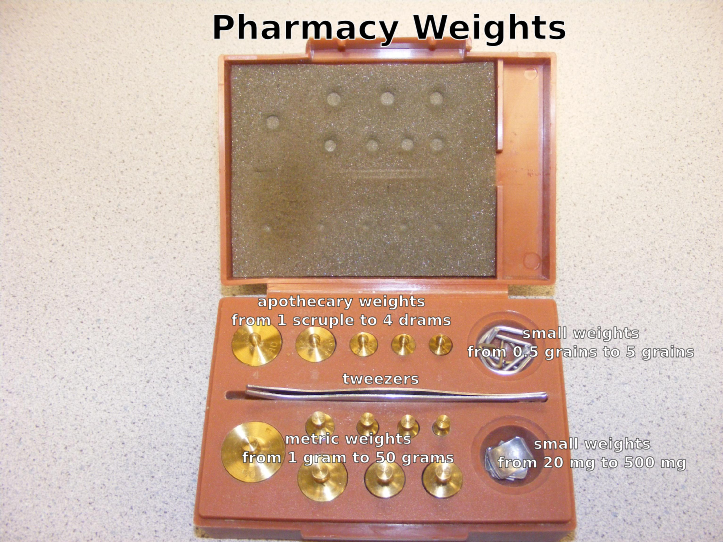
Class A prescription balance (Class III balance)
Pharmacy weights
Electronic balance
Analytical balance
Glassine papers (weighing papers)
Weighing boats (weighing canoes)
Ointment slab or pad
Spatula
Spatula cards
Scoopula
Glass stirring rods
Hot plate
Magnetic stir
Beakers
Graduated cylinder
Erlenmeyer flask
Droppers
Funnels
Oral syringes
Ointment jars and tubes
Prescription vials
Bottles
Processes
Accuracy is a chief concern when it comes to weighing (pharmacy prefers the term weight to mass) and measuring the necessary quantity of ingredients for pharmaceutical compounding. The United States Pharmacopeia (USP) allows a tolerance of plus or minus 5 percent for most preparations.
Weighing
Weighing items may involve a traditional prescription balance along with a set of weights or by more modern means using an electronic and/or analytical balance.
A Class A prescription balance is also known as a double-pan (2-pan) torsion balance; now designated a Class III balance. Class III balances have a minimal weighable quantity (MWQ) of 120 mg and must have a maximum weighable quantity (the capacity) of 60 grams, though some will weigh up to 120 grams; you need to look at the stated capacity of the balance.
The sensitivity (sensitivity requirement) of a balance is the amount of weight that will move balance pointer one-division marker on the marker plate. If you attempt to weigh something less than the balance's sensitivity requirement, the balance will give you a reading of zero, that is, the mass on the balance is too small for it to sense.
A Class III balance has a maximum sensitivity requirement of 6 mg to produce a change of one index division in the rest point as shown by the balance indicator. The smaller the weight required to produce this one division displacement, the greater is the sensitivity of the balance.
Electronic single-pan balances have internal weights, digital display features, and readability and precision of 1 mg; are available at relatively reasonable costs. Most find these balances easier to use and more accurate than a traditional double-pan balance.
Never place weights, drugs, or chemicals directly onto a balance pan. This includes the balance pan of an electronic or analytical balance. Glassine weighing papers or weighing canoes are generally preferred for weighing. Glassine papers have a smooth, shiny surface that does not absorb materials placed on them, and drugs and chemicals are easily slipped off for a complete transfer. The paper should be diagonally creased from each corner or folded in quarters and then flattened and placed on the pans. This helps to contain the substance being weighed and prevents spilling on the balance pans or balance platform. New weighing papers or weighing boats should be used with each new drug to prevent contamination
There are some general rules about using a balance that help to maintain a prescription balance in top condition.
- Always use the balance on a level surface and in a draft free area
- Always cover both pans with weighing papers or use weighing boats. These protect the pans from abrasions, eliminate the need for repeated washing, and reduce loss of drug to porous surfaces
- A clean paper or boat should be used for each new ingredient to prevent cross contamination of components
- The balance must be readjusted after a new weighing paper or boat has been placed on each pan. Weighing papers taken from the same box can vary in weight by as much as 65 mg. Larger weighing boats can vary as much as 200 mg
- Always arrest the balance before adding or removing weight from either pan. *Although the balance is noted for its durability, repeated jarring of the balance will ultimately damage the working mechanism of the balance and reduce its accuracy
- Use a spatula to add or remove ingredients from the balance. Do not pour ingredients out of the bottle
- Always clean the balance, close the lid, and arrest the pans before storing the balance between uses
Electronic and analytical balances have digital displays and may have internal calibration capabilities. These may either be top-loading or encased to protect the balance from dust and drafts. Electronic balances generally have a greater level of accuracy than a prescription balance.
An analytical balance is used to measure mass to a very high degree of precision and accuracy. The measuring pan(s) of a high precision (0.1 mg or better) analytical balance are inside a transparent enclosure with doors so that dust does not collect and so any air currents in the room do not affect the balance's operation. An analytical balance is usually ten-fold more accurate than an electronic balance.
To use either an electronic or analytical balance, follow these guidelines:
- Always use the balance on a level surface in a draft free area
- If the balance has a level bubble, make sure the bubble is inside the bulls eye and make adjustments in the leveling feet as needed
- Place a weighing boat or a single sheet of weighing paper on the pan
- When the balance has determined the final weight, press the tare bar to compensate for the weight of the weighing boat or weighing paper.
- As ingredients are added or removed from the weighing boat, the digital display will show the weight of the ingredient in the boat.
- As ingredients are added or removed from the weighing boat. Make sure the balance has determined the final weight before adding or removing any ingredient
Measuring
Graduates (both cylinders and conical), volumetric flasks (such as Erlenmeyer flasks and beakers), burettes, pipettes, medicine droppers, and syringes are examples of volumetric equipment. The term volumetric simply means measures volume, therefore volumetric equipment is used for measuring volumes of liquid. Volumetric vessels are either to deliver (TD) or to contain (TC). To deliver means that the vessel must be completely emptied to dispense the needed volume. Single volume pipettes, syringes, droppers and small calibrated pipettes are TD vessels. To contain means that the vessel does not need to be completely emptied to dispense the needed volume. Volumetric flasks, graduates, and some calibrated pipettes are TC vessels.
For maximum accuracy in measuring, select a measuring device with a capacity equal to or slightly larger than the volume to be measured. As graduated cylinders are usually more accurate than conical graduates or Erlenmeyer flasks we will focus on them. The general rule is to measure volumes no less than 20% of the capacity of a graduate. For example, 2 mL would be the minimum measurable quantity (MMQ) for a 10 mL graduate. Whenever you are measuring items in a graduated cylinder, you should look at the bottom of the meniscus. The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. Water and oil based substances typically have a concave meniscus.
Sterile compounding
Many facilities find it necessary to provide patient specific compounded sterile preparations including hospitals, surgicenters, ambulatory care facilities, long term care facilities, home infusion companies, nuclear pharmacies, etc. USP Chapter 797, Pharmaceutical Compounding-Sterile Preparations, provides the first set of enforceable sterile compounding standards issued by the United States Pharmacopeia (USP). USP Chapter 797 describes the procedures and requirements for compounding sterile preparations and sets the standards that apply to all settings in which sterile preparations are compounded.
USP 797 is based around three categories of microbial contamination; low-risk level CSPs, medium-risk level CSPs, and high-risk level CSPs. It is important to remember that these risk categories are based solely on contamination risk and do not include any consideration of potential risks to the compounder. There are also additional requirements for compounding hazardous drugs.
Low-risk compounding is performed within an ISO Class 5 (or better) environment and involves the use of no more than three sterile products and no more than two entries through a medication port.
Medium-risk compounding is performed within an ISO Class 5 (or better) environment and involves more complex manipulations of sterile products including more product manipulations and potentially more medication port entries than Low-risk compounding. As long as all products and equipment used directly in compounding it is still considered medium-risk. A facility designed to prepare medium-risk compounds may also prepare low-risk compounds.
High-risk compounding is performed within an ISO Class 5 (or better) environment but involves the use of either nonsterile products and/or equipment. These medication must be terminally sterilized prior to use for a patient. Terminal sterilization methods may include filtration through a 0.2 micron filter, the use of an autoclave, and/or gamma irradiation. A facility designed to prepare high-risk compounds may also prepare low and medium-risk compounds.
Hazardous drugs provide additional safety concerns for the preparer. A facility that prepares more than a low volume of hazardous drugs is required to have a separate negative pressure room in which an appropriate primary engineering control will be housed. Primary engineering controls used to prepare hazardous drugs should not be used to prepare non-hazardous drugs.
Facilities
A facility must have an adequate design to support the risk level of compounding performed within it. The following two images are examples of potential layouts for low and medium-risk level compounding compared to high-risk level compounding and hazardous drug compounding.
Pharmacy personnel will enter through the ante area where they will perform appropriate cleansing and garbing. Non-hazardous medications will then be taken into a positive pressure buffer area and prepared within a primary engineering control such as a laminar airflow workbench (LAFW) or a compounding aseptic isolator (CAI).
If a pharmacy prepares high risk compounds the buffer room and the ante room need to be separate rooms.
If preparing more than a low volume of hazardous drugs, pharmacies will need a separate negative pressure buffer area, which will house a primary engineering control such as a biological safety cabinet (BSC) or a compounding aseptic containment isolator (CACI).
Equipment and supplies
Below is a list of equipment commonly used to prepare CSPs.
Laminar airflow workbench (LAFW)
Compounding aseptic isolator (CAI)
Biological safety cabinet (BSC)
Compounding aseptic containment isolator (CACI)
Ampules
Single dose vial
Multi-dose vial
Sterile powder for injection
Prefilled disposable syringes
Ready-to-mix systems (Add-Vantage, Minibag Plus, etc.)
Dual chamber vial
Minibags
Large volume parenteral
Glass bottle for IV fluids
Duplex bags
Administration sets
Needles
Syringes
Filter needles
Processes
Accuracy of measurement is a primary concern when performing sterile compounding. Measurements are primarily performed using syringes. Depending on the size of the syringe it may have markings as small as 0.01 mL on a 1 cc syringe (commonly called a tuberculin syringe), whereas a 60 mL syringe may only have a marking every 1 mL. This is why it is important to use a syringe that comes closest to the volume that needs to be measured which ideally can also contain the full quantity desired. A 60 cc syringe would not be used to measure 0.25 mL and a 1 cc syringe should not be used to measure 50 mL. Common syringe sizes include 1 cc, 3 cc, 5 cc, 10 cc, 20 cc, 30 cc, and 60 cc.
Calculations often used for compounding
Pharmacy compounding often requires pharmacists and technicians to perform various calculations in order to prepare a final product. In the following section we will review common conversions, common problem solving methods such as ratio proportions and dimensional analysis, and look at various scenarios involving compounding and calculations.
As this chapter section is only intended as review, if you want longer explanation about these calculation concepts look at USP 1160 Pharmaceutical Calculations in Prescription Compounding (this chapter can be found online via a search engine) or for an even more in depth explanation with numerous practice problems look at Pharmaceutical Calculations by Sean Parsons (this can be downloaded for free at http://pharmaceuticalcalculations.org/).
Conversions
With respect to conversions, it makes most sense to review the English (or household) system, the metric system, and common conversions between the two.
English/household system
Weight
16 ounces (oz) = 1 pound (lb)
Volume
3 teaspoons (tsp) = 1 tablespoon (Tbs)
2 tablespoons (Tbs) = 1 fluid ounce (fl oz)
8 fluid ounces (fl oz) = 1 cup
16 fluid ounces (fl oz) = 1 pint (pt)
Length
12 inches (in) = 1 foot
Metric system
Weight
1000 nanograms (ng) = 1 microgram (mcg)
1000 micrograms (mcg) = 1 milligram (mg)
1000 milligrams (mg) = 1 gram (g)
1000 grams (g) = 1 kilogram (kg)
Volume
1 cubic centimeter (cc) = 1 milliliter (mL)
1000 milliliters (mL) = 1 liter (L)
Length
1000 millimeters (mm) = 1 meter (m)
100 centimeters (cm) = 1 meter (m)
Conversions between English/household system and metric system
Weight
1 ounce (oz) = 28.4 grams (g)
1 pound (lb) = 454 grams (g)
1 kilogram (kg) = 2.2 pounds (lbs)
Volume
1 teaspoon (tsp) = 5 milliliters (mL)
1 tablespoon (Tbs) = 15 milliliters (mL)
1 fluid ounce (fl oz) = 30 milliliters (mL)
1 pint (pt) = 480 milliliters (mL)
Length
1 inch (in) = 2.54 centimeters (cm)
Ratio proportion
A ratio proportion is a statement of equality between two ratios. An example would be:
Since the ratios are equal, if there is a missing piece of data (a variable that needs to be solved for), you can cross multiply and divide to find the value of the missing data. The labels also cross multiply and cancel out as appropriate. When cross multiplying to solve for a variable, multiply the numbers that are diagonal to each other and then divide by the number diagonal to the variable.
Example: Magnesium sulfate is available as 5 grams per 10 milliliters, so how many milliliters will you need for a dose of 2 grams?
Notice that the grams canceled each other out since they divided into each other.
Dimensional analysis
Dimensional analysis (also called factor label) involves multiplying a series of fractions in such a way that units are canceled out till you are left for the units you are looking for.
Example: To demonstrate this concept we will look at how many fluid ounces are in 2 liters:
Percentage strength
There are many ways to express the concentration of a drug; one of the most common is percentage strength. There are three kinds of percentage strength that you will frequently use when doing dosage calculations:
weight/weight percent (w/w%) - typically measured in grams/100 grams and include items like ointments and creams
volume/volume percent (v/v%) - typically measured in milliliters/100 milliliters and include items like alcohol preparations and oil-in-water preparations
weight/volume percent (w/v%) - measured in grams/100 milliliters and includes drugs dissolved in solution
An additional item to help you solve percentage strength problems when setting them up as ratio proportions is to think of it as 'active ingredient over mixture'.
The following are some examples of percentage strength.
Example: What would be the weight, expressed in grams, of zinc oxide (zinc oxide is the active ingredient) in 120 grams of a 10% zinc oxide ointment (the ointment is the total mixture)?
Example: How many milliliters of isopropyl alcohol are in a 480 milliliter bottle of 70% isopropyl alcohol?
Example: If 4.5 grams of a medication is dissolved in 2 fluid ounces of solution, what is the percentage strength (you will need to convert the fluid ounces to milliliters before solving this problem)?
Diluting stock solutions
A stock solution is a concentrated solution from which less concentrated solutions may be prepared. The stock solution diluted with a diluent (sometimes also called a solvent), which may be water or some other suitable substance. Whenever a dilution is required, the necessary math may be performed up to three different ways depending on what information is present including a series of ratio proportions, the dilution formula, or the alligation method. The following example will be solved all three ways.
Example: If 500 milliliters of a 5% stock solution is ordered, how many milliliters of a 25% stock solution is needed to prepare the desired solution and how many milliliters of a suitable diluent are required?
Series of ratio proportions
Solving this with a series of ratio proportions requires the least explanation as the concept has already been explained earlier in this chapter. To solve the problem this way you would first need to determine how much drug is actually needed for the desired solution and then figure out how many milliliters of stock solution will be needed to provide the necessary quantity of drug. Lastly, you can subtract the milliliters of stock solution required from the final volume to determine the quantity of diluent needed.
Solving this with the dilution formula requires you to plug information into the following formula and solve for the variable. Once you know both the final volume and the stock solution volume, you can subtract the milliliters of stock solution required from the final volume to determine the quantity of diluent needed.
Dilution formula
(C1)(Q1)=(C2)(Q2)
C1 = concentration of available stock solution (percentage strength is a type of concentration)
Q1 = quantity of available stock solution needed
C2 = concentration of ordered solution
Q2 = quantity of ordered solution needed
So, if we plug the numbers from our example problem into this formula and solve it looks like this:
Alligation method
The alligation method provides another way to solve this problem and may also be useful when you are mixing two different stock solutions together in order to get a third concentration. The following is an image with a brief description of how to set a problem up this way.
- Place the highest concentration in the upper left-hand corner
- Place the lowest concentration in the lower left-hand corner (this will often be a diluent which will have a 0% concentration)
- Place the desired concentration in the center
- Find the difference between the highest concentration and the desired concentration to find the parts of lowest concentration
- Find the difference between the lowest concentration and the desired concentration to find the parts of highest concentration
- Add the parts of highest concentration and the parts of lowest concentration to find the total parts
- This provides you with a ratio that you can use to finish solving the problem.
With those concepts in mind, let's plug numbers in from our example question.
Mixing liquid preparations
Sometimes you will need to determine how much to use of various products to fulfill a recipe written by a physician, sometimes you may take a recipe for a liquid medication and modify it for a different final volume, and other times you may need to either open up capsules or crush tablets and dissolve or suspend them in a liquid vehicle. Let's look at an example of each scenario.
Example: How much clindamycin phosphate (the stock vial concentration is 150 mg/mL) and how much Cetaphil Lotion are needed to make the following compound?
Rx clindamycin phosphate 1200 mg in Cetaphil Lotion
Disp: 120 mL
Sig: aa hs ud
First, we should figure out how many mL of the clindamycin phosphate stock solution we should use:
Then, we should figure out how much Cetaphil Lotion we'll need to make this 120 mL:
Example: A prescription is written for a mouthwash containing 170 mL diphenhydramine elixir, 50 mL lidocaine viscous, 200 mL nystatin suspension, 52 mL erythromycin ethyl succinate suspension, and 28 mL of cherry syrup to make 500 mL of mouthwash. How much of each ingredient would be needed if you only wanted to prepare 240 mL of the mouthwash?
First, make a ratio comparing each ingredient and specifying the total volume:
170:50:200:52:28 to make 500 mL mouthwash
Then, solve for how much of each ingredient is needed to make 240 mL of mouthwash:
Example: How many 25 mg tablets of metoprolol tartrate and how many milliliters of Ora-Plus and Ora Sweet are needed to compound the following prescription?
Rx metoprolol tartrate 6.25 mg/tsp in a 50:50 mixture of Ora-Plus and Ora-Sweet
Disp: 300 mL
Sig: i tsp po bid
First, determine how many metoprolol tartrate tablets are needed:
You will often expect the powder volume from crushed tablets and opened capsules to be negligible, but since we don't know exactly, we will simply do the math for both liquids as if all the volume was from our two suspending agents. Since they are a 50:50 mixture it means that we only need half the volume for each suspension.
Compounding ointments gels and creams
Sometimes compounding a semi-solid mixture (ointment, gel, or cream) can be as straight forward as mixing two semi-solids together. Other times it may require incorporating a medication into a semi-solid base. Let's look at an example of each.
Example: A prescription is written for equal parts triamcinolone 0.1% cream and Lamisil cream, dispense 30 grams. How many grams of triamcinolone 0.1% cream are needed to fill the prescription? How many grams of Lamisil cream are needed to fill the prescription? What is the final percentage strength of triamcinolone in the compound?
To solve this we need to first recognize that the ratio between the ingredients is 1:1 for a total of 2 parts. With that in mind, we know that half the total weight is how many grams of each ingredient we'll need.
Therefore, we will need 15 g of triamcinolone 0.1% cm and 15 g of Lamisil cm.
Next, we need to evaluate the final percentage strength of triamcinolone in the compound. There are 2 ways to do it, one is to calculate just how much triamcinolone is in the mixture and then figure out its percentage strength, the other is to also divide by 2 like we did the total weight. Both ways will be demonstrated, but recognize that you only have to do it one way to achieve the correct answer.
or
0.1% ÷ 2 = 0.05% triamcinolone
Obviously the second way was easier, but it is good to know that you will get the same answer either way.
It is also noteworthy that the methodology used in this example will apply to any compounding problem where you are mixing ingredients in equal parts.
Example: If 50 g of salicylic acid ointment contains 10 grams of salicylic acid, what is the percentage strength of salicylic acid in the ointment?
This problem is just a simple w/w percentage strength problem:
Reconstituting powders
Some medications come from the manufacturer as a lyophilized powder in order to provide them with a longer shelf life. Some medications have a negligible powder volume and others have a more significant volume. Whenever looking at a powder that has been reconstituted, you must be aware of the resulting concentration of the medication, whether it is an oral medication labeled as 400 milligrams per teaspoon or injectable medication with a concentration of 250,000 units per milliliter. Let's look at an example with respect to a powder intended for reconstitution.
Example: If a 20 gram vial of cefazolin Na is reconstituted with 192 milliliters of sterile water and contains 8 milliliters of powder volume, how many milliliters of the reconstituted solution will be needed to provide a 1 gram dose?
Dosages based on body weight
Many drugs need to be calculated based on body weight. Some of the drugs where you will see this most often include chemotherapy, steroids, antibiotics, heparinoids, and drugs for pediatric and geriatric patients. If a medication is to be based on body weight it will usually be requested in mg/kg. Look at the following example:
Example: A physician orders cyclophosphamide to be given 5 mg/kg in a 50 mL D5W bag. If the patient weighs 132 lbs and the concentration of the drug after reconstitution is 500 mg/10 mL, how many milliliters will you need to withdraw from the vial and add to the bag in order to prepare this admixture.
Dosages based on body surface area
Body surface area (BSA) is the measured or calculated surface of the human body, and it is measured in square meters (m2). For many clinical purposes BSA is a better indicator of metabolic mass than body weight because it is less affected by abnormal adipose mass. There are a number of weighs to calculate, but one of the simplest is the Mosteller formula.
Mosteller
Example: If a patient is 6' 1" tall and weighs 176 lbs, what is the patient's BSA?
Example: A physician orders bleomycin in a dose of 20 units/m2 for a 5'3" patient that weighs 110 lbs. How many units of bleomycin should the patient receive?
old portion
The art of pharmaceutical compounding has ancient roots dating back to early hunter gatherer societies. These ancient civilizations utilized pharmaceutical compounding for religion, grooming, keeping the healthy well, treating the ill and preparing the dead. These ancient compounders produced the first oils from plants and animals. They discovered poisons and the antidotes. They made ointments for wounded patients as well as perfumes for customers.
Today compounding is still a necessary skill for many pharmacists and pharmacy technicians. Extemporaneous compounding can be defined as the preparation, mixing, assembling, packaging, and labeling of a drug product based on a prescription from a licensed practitioner for the individual patient in a form that the drug is not readily available in (extemporaneous = impromptu, compounding = the act of combining things). Extemporaneous compounding is required for prescription orders that are not commercially available in the requested strength or dosage form.
There are two types of pharmaceutical compounding, sterile (ophthalmics, IVs, parenteral nutrition, chemotherapy, and various other injectables) and nonsterile (creams, ointments, oral suspensions, capsules, suppositories, medication sticks, troches, etc.). This Entry will primarily focus on nonsterile compounding.
Categories of nonsterile compounding
USP Chapter <1075> has designated three categories of compounding related to different levels of required experience, training, and physical facilities. The considerations factoring into this are:
- the difficulty/complexity of the compound,
- product stability and warnings,
- packaging and storage requirements,
- the physical dosage forms,
- the complexity of calculations required,
- local versus systemic effects of the product,
- risk to the compounder, and
- risk to the patient.
These categories are broken into Simple, Moderate, and Complex compounding. Simple compounding involves preparing compounds from USP monographs and peer-reviewed journals or even just reconstituting and manipulating commercial products within manufacturer guidelines. Required procedures and equipment are well documented. Moderate compounding requires special calculations and procedures and is generally not as well documented as Simple compounding. Complex compounding requires special training, environment, facilities, equipment, and procedures.
Basic steps for preparing compounded drug preparations
Regardless of the compounding category, there is a general order of work for all extemporaneous compounding. By following a procedure you will be able to work more effectively.
- Carefully read and interpret the prescription or medication order.
- Note any missing or confusing information; clarify, gather, and add this information to the drug order.
- Check the dose,dosage regimen, dosage form, and route of administration for appropriateness.
- Determine a preliminary compounding procedure.
- Perform necessary calculations. If possible have a colleague check calculations.
- Select required ingredients. It is the responsibility of the pharmacist to choose appropriate quality ingredients for compounding. The preferred grade for this is USP or NF. If an official USP or NF ingredient is not available, the pharmacist should use professional judgment in the selection of an alternative source so that the safety and purity of the ingredient is assured. This may require requesting a certificate of analysis from the supplier.
- Choose appropriate compounding equipment.
- Using recommended techniques, prepare the product.
- A visual inspection of the product should be done.
- Choose an appropriate container and package the preparation.
- Determine an appropriate expiration date (beyond-use date)
- Label container and include recommended auxiliary labels
- Document the compounding process.
Legal issues
As explained in the Food and Drug Administration Modernization Act, there is a special exemption to ensure continued availability of compounded drug products prepared by pharmacists to provide patients with individualized therapies not available commercially. The lack of commercial availability is the primary determining factor as to whether or not a pharmacy is legally allowed to compound a medication that a physician has written for.
The legal duties of the pharmacy technician are to perform nonjudgmental technical duties associated with the preparation and distribution of prescription drugs, provided that a pharmacist verifies the accuracy of all acts, tasks, and functions performed by the technician and that the technician functions under the direct supervision of and is responsible to a pharmacist. Accurate record keeping can be facilitated by a pharmacy technician who can maintain a control log of all ingredients, lot numbers, and amounts used in compounded preparations, as well as assigned prescription numbers.
The pharmacist is responsible for the actions of the technician and the quality of each preparation. The pharmacist must evaluate the preparation of the compounded prescription as it is prepared by the pharmacy technician and must review that prescription before it is dispensed.
Lab safety
The vast majority of chemicals used in compounding have a relatively low safety risk, but that does not mean that all items used in compounding have little or no risk. There are several things that should be kept in mind when compounding:
- Personal Protective Equipment (PPE)
- gloves
- goggles or face shield
- lab coats or gowns
- masks or respirators
- ventilation hoods
- Eye Wash Station
- Material Safety Data Sheets (MSDS)
- Initial incident reports in case of an injury (no matter how minor)
Equipment
Below is a list of equipment commonly used for extemporaneous compounding followed by a set of images.
- Mortar and pestle
- Class A prescription balance (Class III balance)
- Pharmacy weights
- Electronic balance
- Analytical balance
- Glassine papers (weighing papers)
- Weighing boats (weighing canoes)
- Ointment slab or pad
- Spatula
- Spatula cards
- Scoopula
- Glass stirring rods
- Hot plate
- Magnetic stir
- Beakers
- Graduated cylinder
- Erlenmeyer flask
- Droppers
- Funnels
- Oral syringes
- Ointment jars and tubes
- Prescription vials
- Bottles
Weighing and measuring
Accuracy is a chief concern when it comes to weighing (pharmacy prefers the term weight to mass) and measuring the necessary quantity of ingredients for pharmaceutical compounding. The United States Pharmacopeia (USP) allows a tolerance of plus or minus 5 percent for most preparations.
Weighing
Weighing items may involve a traditional prescription balance along with a set of weights or by more modern means using an electronic and/or analytical balance.
A Class A prescription balance is also known as a double-pan (2-pan) torsion balance; now designated a Class III balance. Class III balances have a minimal weighable quantity (MWQ) of 120 mg and must have a maximum weighable quantity (the capacity) of 60 grams, though some will weigh up to 120 grams; you need to look at the stated capacity of the balance.
The sensitivity (sensitivity requirement) of a balance is the amount of weight that will move balance pointer one-division marker on the marker plate. If you attempt to weigh something less than the balance's sensitivity requirement, the balance will give you a reading of zero, that is, the mass on the balance is too small for it to sense.
A Class III balance has a maximum sensitivity requirement of 6 mg to produce a change of one index division in the rest point as shown by the balance indicator. The smaller the weight required to produce this one division displacement, the greater is the sensitivity of the balance.
Electronic single-pan balances have internal weights, digital display features, and readability and precision of 1 mg; are available at relatively reasonable costs. Most find these balances easier to use and more accurate than a traditional double-pan balance.
Weighing papers and weighing boats
Never place weights, drugs, or chemicals directly onto a balance pan. This includes the balance pan of an electronic or analytical balance. Gassine weighing papers or weighing canoes are generally preferred for weighing. Glassine papers have a smooth, shiny surface that does not absorb materials placed on them, and drugs and chemicals are easily slipped off for a complete transfer. The paper should be diagonally creased from each corner or folded in quarters and then flattened and placed on the pans. This helps to contain the substance being weighed and prevents spilling on the balance pans or balance platform. New weighing papers or weighing boats should be used with each new drug to prevent contamination
Prescription balance and weights
There are some general rules about using a balance that help to maintain a prescription balance in top condition.
- Always use the balance on a level surface and in a draft free area
- Always cover both pans with weighing papers or use weighing boats. These protect the pans from abrasions, eliminate the need for repeated washing, and reduce loss of drug to porous surfaces
- A clean paper or boat should be used for each new ingredient to prevent cross contamination of components
- The balance must be readjusted after a new weighing paper or boat has been placed on each pan. Weighing papers taken from the same box can vary in weight by as much as 65 mg. Larger weighing boats can vary as much as 200 mg
- Always arrest the balance before adding or removing weight from either pan. *Although the balance is noted for its durability, repeated jarring of the balance will ultimately damage the working mechanism of the balance and reduce its accuracy
- Use a spatula to add or remove ingredients from the balance. Do not pour ingredients out of the bottle
- Always clean the balance, close the lid, and arrest the pans before storing the balance between uses
The following are steps for how to use a prescription balance:
- Arrest the balance by turning the arrest knob. Level the balance (front to back) by turning the leveling screw feet all the way into the balance and then moving them the same direction until the 4 sides of the balance are equidistant from the bench. For balances with leveling bubbles, move the leveling screw feet until the bubble is in the middle of the tube.
- Set the internal weights to zero. This is done by turning the calibrated dial to zero.
- Level the balance (left to right) by adjusting the leveling screw feet. To shift the pointer left, grasp both the screw feet between the thumbs and forefingers and rotate so that thumbs move inward. To shift the pointer to the right, rotate both screw feet so that forefingers move toward back of the balance. Continue adjusting the screw feet slowly until the pointer rests at the center of the index or swings equal distances to the right and left of the center.
- Place one weigh boat on each weighing pan. The small weigh boats will hold up to 2 g., while the larger weigh boats will hold up to 30 g.
- Unlock the balance by releasing the arrest knob and note the rest point of the pointer on the index. If the pointer does not rest at the center of the index, then it will be necessary to level the balance left to right.
- Arrest the balance and place the required weights on the right pan. Place the material to be weighed on the left pan.
- Release the balance and note the shift of the pointer on the index. If the pointer shifts left, too much of the substance is on the pan and a portion should be removed. If it shifts right, there is too little of the substance and more should be added. Using a spatula, remove or add material; arrest the balance each time before a transfer is made.
- Once you are satisfied that you have made an accurate measurement, double check to make sure that you have weighed the correct substance (check the label) and that you have used the correct weights (internal and external).
Electronic balance and analytical balance
Electronic and analytical balances have digital displays and may have internal calibration capabilities. These may either be top-loading or encased to protect the balance from dust and drafts. Electronic balances generally have a greater level of accuracy than a prescription balance.
An analytical balance is used to measure mass to a very high degree of precision and accuracy. The measuring pan(s) of a high precision (0.1 mg or better) analytical balance are inside a transparent enclosure with doors so that dust does not collect and so any air currents in the room do not affect the balance's operation. An analytical balance is usually ten-fold more accurate than an electronic balance.
To use either balance, follow these guidelines:
- Always use the balance on a level surface in a draft free area
- If the balance has a level bubble, make sure the bubble is inside the bulls eye and make adjustments in the leveling feet as needed
- Place a weighing boat or a single sheet of weighing paper on the pan
- When the balance has determined the final weight, press the tare bar to compensate for the weight of the weighing boat.
- As ingredients are added or removed from the weighing boat, the digital display will show the weight of the ingredient in the boat.
- As ingredients are added or removed from the weighing boat. Make sure the balance has determined the final weight before adding or removing any ingredient
Measuring
Graduates (both cylinders and conical), volumetric flasks (such as Erlenmeyer flasks and beakers), burettes, pipettes, medicine droppers, and syringes are examples of volumetric equipment. The term volumetric simply means measures volume, therefore volumetric equipment is used for measuring volumes of liquid. Volumetric vessels are either to deliver (TD) or to contain (TC). To deliver means that the vessel must be completely emptied to dispense the needed volume. Single volume pipettes, syringes, droppers and small calibrated pipettes are TD vessels. To contain means that the vessel does not need to be completely emptied to dispense the needed volume. Volumetric flasks, graduates, and some calibrated pipettes are TC vessels.For maximum accuracy in measuring, select a measuring device with a capacity equal to or slightly larger than the volume to be measured. As graduated cylinders are usually more accurate than conical graduates or Erlenmeyer flasks we will focus on them. The general rule is to measure volumes no less than 20% of the capacity of a graduate. For example, 2 mL would be the minimum measurable quantity (MMQ) for a 10 mL graduate. Whenever you are measuring items in a graduated cylinder, you should look at the bottom of the meniscus. The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. Water and oil based substances typically have a concave meniscus as demonstrated in the image on the right.
Definitions
trituration
- A method to reduce particle size (comminution); it may also include the grinding together of two or more substances in a mortar to mix them as you should want a fine powder to make incorporation better. Trituration is achieved by firmly holding the pestle and exerting a downward pressure with it while moving it in successively larger circles starting at the center of the mortar, moving outward to the side of the mortar, then back again toward the center.
spatulation
- The mixing of powders on an ointment pad or slab using a spatula. With this method there is no particle size reduction, so the powders to be mixed must be fine and of uniform size.
geometric dilution or geometric incorporation
- Geometric dilution is the process by which a homogenous mixture of even distribution of two or more substances is achieved. When using this method, the smallest quantity of active ingredient is mixed thoroughly with a proportion quantity of the diluent or base on the ointment slab. More diluient (base) is added in amounts proportionate to the volume of the mixture on the ointment slab. This process is repeated until all of the ingredients are incorporated in the mixture.
levigation
- The process of reducing particle size of a solid by triturating it in a mortar or spatulating it on an ointment slab or pad with a small amount of a liquid called a levigating agent. Levigating agents make incorporating solids easier, and they make a smooth, elegant preparation.
- Choose a levigating agent (the liguid) similar to the ointment base. For example, mineral oil is the levigating agent of choice for oily bases. Glycerin, which is mixable with water, is usually used for water-soluble bases. Often the ointment formulation contains an ingredient that can be used as a levigating agent. This is the preferred situation.
- The prescription order may not have the levigating agent listed, therefore use the minimum amount of levigating agent necessary to lubricate the powders. The amount used depends on the amount of powder to be levigated. Unless the prescription calls for a given amount of levigating agent, the minimum amount is recommended. You need to note this on the prescription order or compounding log. The formula may already contain a liquid that is a suitable levigating agent that liquid should be used.
- A levigating agetn is NOT needed when:
- the solid being incorporated has a very fine particle size,
- the quantity of solid to incorporate is small,
- the ointment base is soft,
- the final product is to be a stiff ointment or paste.
Compounded dosage forms
Compounding pharmacies are capable of preparing a broad array of dosage forms including capsules, tablets, oral suspensions and solutions, suppositories, troches (lozenges), various topical dosage forms (gels, lotions, ointments, creams), lollipops, and more. One of the things that tends to make compounding both interesting and difficult is finding the best way to manipulate a dosage form to benefit a patient.
Labeling
Product labels for extemporaneous compounds must follow all federal and provincial requirements. Necessary items to include on the label are:
- the name of the preparation;
- the internal identification number;
- the beyond-use date
- the initials of the compounder who prepared the product;
- any storage requirements; and
- any other statements required by law.
Beyond-use date
The requirement to provide a beyond-use date sometimes causes confusion as there is some ambiguity between what an expiration date is and what a beyond-use date is. An expiration date is the date put on the label of a drug product by the manufacturer or distributor of the product. Federal law requires that manufactured (compounding is not considered manufacturing) drug products be labeled with an expiration date. The beyond-use date is the date placed on a prescription bottle by a pharmacy noting when that prescription should no longer be used. The AMA states, "The beyond-use date placed on the label shall be no later than the expiration date on the manufacturer’s container. The beyond-use date is a date after which an article [drug] must not be used. Based on the information supplied by the manufacturer, the dispenser shall place on the label of the prescription container a suitable beyond-use date to limit the patient’s use of the article."
For water containing formulations, prepared from ingredients in solid form, the beyond use date is not later than 14 days when stored at cold temperatures. Generally, a two-week dating is the maximum for ointments containing water if a preservative is not present. Ointment with water are subject to microbial growth. Ointments without water can have much longer dating. Ointment jars, although widely used, expose the preparation to air and microbial contamination when opened and when the ointment is removed using the fingers. The maximum beyond-use date for a very stable preparation would be 6 months or 25% of the time remaining between the time of compounding and the shortest expiration date of the ingredients, whichever is earlier. You need to consult available literature if possible and professional judgment is required in these cases. For all other formulations, the beyond-use date is not later than the intended duration of therapy or 30 days, whichever is earlier.
See also
Food and Drug Administration Modernization Act
References
- Tech Lectures For the Pharmacy Technician, Section XXIV - Principles of Compounding, http://www.tlectures.com/compounding.pdf
- U.S. Pharmacopeia 29th Edition, USP <795> - Pharmaceutical Compounding - Nonsterile Preparations, http://www.pharmacopeia.cn/v29240/usp29nf24s0_c795.html
- About.com, Are Beyond-Use Dates Different Than Expiration Dates?, January 16, 2011, http://bipolar.about.com/od/medications/a/beyond_use.htm
- Ontario College of Pharmacists, Guidelines for Compounding Preparations, http://www.ocpinfo.com/client/ocp/OCPHome.nsf/web/Guidelines+to+Compounding+Preparations
- Practical Guide to Contemporary Pharmacy Practice Third Edition, Judith Thompson, LWW, January 16, 2009, ISBN: 9780781783965
- International Journal of Pharmaceutical Compounding, Basics of Compounding: Implementing United States Pharmacopeia Chapter <795> Pharmaceutical Compounding-Nonsterile Preparations, Part 1, Loyd V Allen, Jr., PhD RPh, Vol. 15 No. 4, July/August 2011