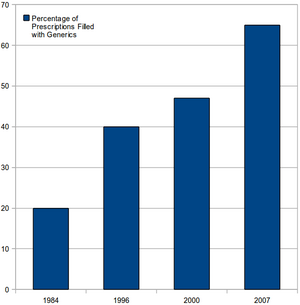
Drug Price Competition and Patent-Term Restoration Act
From Rx-wiki
Hatch-Waxman amended the Federal Food, Drug, and Cosmetic Act. It sets forth the process by which would-be marketers of generic drugs can file Abbreviated New Drug Applications (ANDAs) to seek FDA approval of the generic. It allows 180 day exclusivity to companies that are the "first-to-file" an ANDA against holders of patents for branded counterparts.
The FDA will consider a generic product equivalent to a brand drug when both the rate and extent of absorption are not significantly different when administered in the same dose of therapeutic ingredient under similar experimental conditions in studies. The FDA has established specific statistical standards to make a determination of bioequivalence.
The Hatch-Waxman Act grants generic manufacturers standing to mount a validity challenge without incurring the cost of entry or risking enormous damages flowing from any possible infringement. Hatch-Waxman essentially redistributes the relative risk assessments and explains the flow of settlement funds and their magnitude. Hatch-Waxman gives generics considerable leverage in patent litigation: the exposure to liability amounts to litigation costs, but pales in comparison to the immense volume of generic sales and profits.
The other significant factor affecting the pharmaceutical industry was an increase in the length of time for a drug patent to expire from 12 years to 17 years (since then the URAA has extended it to 20 years). This extension was intended to off-set the time it took for a drug to be approved to provide adequate time for the original innovator companies to recoup their initial research investments and make a profit.
See also
Federal pharmacy law
Food and Drug Administration
Orange Book
References
- Pharmacy Times, A Review of Federal Legislation Affecting Pharmacy Practice, Virgil Van Dusen , RPh, JD and Alan R. Spies , RPh, MBA, JD, PhD, https://secure.pharmacytimes.com/lessons/200612-01.asp
- Strauss's Federal Drug Laws and Examination Review, Fifth Edition (revised), Steven Strauss, CRC Press, 2000
- Food and Drug Administration, Legislation, http://www.fda.gov/RegulatoryInformation/Legislation/default.htm
- Food and Drug Administration, History, http://www.fda.gov/AboutFDA/WhatWeDo/History/default.htm
- Authorized Generics: Good or Bad, Pharmacy Times, July 2006, http://www.pharmacytimes.com/issue/pharmacy/2006/2006-07/2006-07-5680
- Generic Epilepsy Drug Switch Tied to Seizures, WebMD, Dec. 6, 2004, http://www.webmd.com/epilepsy/news/20041206/generic-epilepsy-drug-switch-tied-to-seizures
- Dangerous Side Effects, Washington Post, July 16, 2009, http://www.washingtonpost.com/wp-dyn/content/article/2009/07/15/AR2009071503378.html
- Estimated Dates of First Time Generics, Medco, Feb 5, 2010, http://www.google.com/url?sa=t&source=web&cd=2&ved=0CBsQFjAB&url=http%3A%2F%2Fwww.medcohealth.com%2Fmedco%2Fcorporate%2Fhome.jsp%3FltSess%3Dy%26articleID%3DCorpAnticipatedFirstTimeGenericsPDF&ei=vABkTLXcOMP-8AbkleGwCg&usg=AFQjCNHJIuCKU5woVnGLG64tBTZNwNbLkg
- Generic News, Pharmacy Times, December 13, 2010, http://pharmacytimes.com/issue/pharmacy/2010/December2010/GenericNews-1210
- Orange Book Annual Preface, http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm079068.htm