Medicaid Tamper-Resistant Prescription Law
From Rx-wiki
Revision as of 05:10, 3 June 2011 by Kgambridge (Talk | contribs)
The Medicaid Tamper-Resistant Prescription Pad Law was passed in late July as part of the U.S. Troop Readiness, Veterans' Care, Katrina Recovery and Iraq Accountability Appropriations Act of 2007. This law requires physicians and pharmacists to use either electronic prescriptions or tamper-resistant prescription pads for their Medicaid patients. To comply, prescription pads were required to contain at least one tamper resistant feature by April 1, 2008 and since October 1, 2008 all Medicaid scripts must contain one or more industry recognized features from each of three categories of security as specified by the Center for Medicare and Medicaid Service (CMS) .
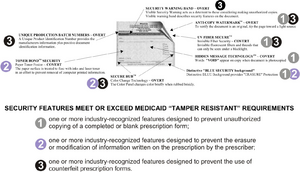
Category One ~ One or More industry-recognized features designed to prevent unauthorized copying of a completed or blank prescription form.
- Void Pantograph background (Hidden Message Technology)
- Reverse Rx Symbol
- Micro Printing
- Artificial Watermark on back of script
- Coin Activated Ink
Category Two ~ One or More industry-recognized features designed to prevent the erasure or modification of information written on a prescription by the prescriber.
- Colored Shaded Pantograph background
- Toner Grip Security Coating
- "Check and Balance" printed features such as "quantity" check boxes, and space to indicate "number of medications" written on prescription form
Category Three ~ One or More industry-recognized features designed to prevent the use of counterfeit prescription forms.
- Security Feature Warning Box and Warning Bands
- Security Back Printing
- Coin Activated Validation
- Batch Number identification
- Secure Rub Color Change Ink
- Consecutive Numbering